In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents
- PMID: 15577941
- PMCID: PMC544906
- DOI: 10.1038/sj.emboj.7600493
In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents
Erratum in
- EMBO J. 2005 Apr 6;24(7):1489
Abstract
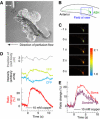
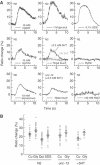
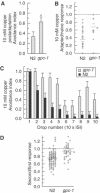
ASH sensory neurons are required in Caenorhabditis elegans for a wide range of avoidance behaviors in response to chemical repellents, high osmotic solutions and nose touch. The ASH neurons are therefore hypothesized to be polymodal nociceptive neurons. To understand the nature of polymodal sensory response and adaptation at the cellular level, we expressed the calcium indicator protein cameleon in ASH and analyzed intracellular Ca(2+) responses following stimulation with chemical repellents, osmotic shock and nose touch. We found that a variety of noxious stimuli evoked strong responses in ASH including quinine, denatonium, detergents, heavy metals, both hyper- and hypo-osmotic shock and nose touch. We observed that repeated chemical stimulation led to a reversible reduction in the magnitude of the sensory response, indicating that adaptation occurs within the ASH sensory neuron. A key component of ASH adaptation is GPC-1, a G-protein gamma-subunit expressed specifically in chemosensory neurons. We hypothesize that G-protein gamma-subunit heterogeneity provides a mechanism for repellent-specific adaptation, which could facilitate discrimination of a variety of repellents by these polymodal sensory neurons.
Figures



References
-
- Bargmann CI, Mori I (1997) Chemotaxis and thermotaxis. In C. elegans II, Riddle DL, Bluementhal T, Mayer BJ, Priess JR (eds) pp 717–737. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press - PubMed
-
- Bargmann CI, Thomas JH, Horvitz HR (1990) Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol 55: 529–538 - PubMed
-
- Boyer JL, Waldo GL, Evans T, Northup JK, Downes CP, Harden TK (1989) Modification of AlF-4- and receptor-stimulated phospholipase C activity by G-protein beta gamma subunits. J Biol Chem 264: 13917–13922 - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

