Cardiac adenoviral S100A1 gene delivery rescues failing myocardium
- PMID: 15578088
- PMCID: PMC529280
- DOI: 10.1172/JCI21454
Cardiac adenoviral S100A1 gene delivery rescues failing myocardium
Abstract
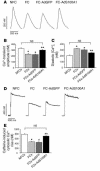
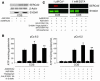
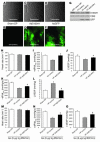
Cardiac-restricted overexpression of the Ca2+-binding protein S100A1 has been shown to lead to increased myocardial contractile performance in vitro and in vivo. Since decreased cardiac expression of S100A1 is a characteristic of heart failure, we tested the hypothesis that S100A1 gene transfer could restore contractile function of failing myocardium. Adenoviral S100A1 gene delivery normalized S100A1 protein expression in a postinfarction rat heart failure model and reversed contractile dysfunction of failing myocardium in vivo and in vitro. S100A1 gene transfer to failing cardiomyocytes restored diminished intracellular Ca2+ transients and sarcoplasmic reticulum (SR) Ca2+ load mechanistically due to increased SR Ca2+ uptake and reduced SR Ca2+ leak. Moreover, S100A1 gene transfer decreased elevated intracellular Na+ concentrations to levels detected in nonfailing cardiomyocytes, reversed reactivated fetal gene expression, and restored energy supply in failing cardiomyocytes. Intracoronary adenovirus-mediated S100A1 gene delivery in vivo to the postinfarcted failing rat heart normalized myocardial contractile function and Ca2+ handling, which provided support in a physiological context for results found in myocytes. Thus, the present study demonstrates that restoration of S100A1 protein levels in failing myocardium by gene transfer may be a novel therapeutic strategy for the treatment of heart failure.
Figures







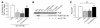
Similar articles
-
S100A1: a regulator of myocardial contractility.Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13889-94. doi: 10.1073/pnas.241393598. Proc Natl Acad Sci U S A. 2001. PMID: 11717446 Free PMC article.
-
S100A1 gene therapy preserves in vivo cardiac function after myocardial infarction.Mol Ther. 2005 Dec;12(6):1120-9. doi: 10.1016/j.ymthe.2005.08.002. Epub 2005 Sep 15. Mol Ther. 2005. PMID: 16168714
-
Targeting phospholamban by gene transfer in human heart failure.Circulation. 2002 Feb 26;105(8):904-7. doi: 10.1161/hc0802.105564. Circulation. 2002. PMID: 11864915 Free PMC article.
-
Targeting Ca2+ cycling proteins and the action potential in heart failure by gene transfer.Basic Res Cardiol. 2002;97 Suppl 1:I136-45. doi: 10.1007/s003950200042. Basic Res Cardiol. 2002. PMID: 12479247 Review.
-
Calcium cycling proteins and force-frequency relationship in heart failure.Basic Res Cardiol. 1996;91 Suppl 2:17-22. doi: 10.1007/BF00795357. Basic Res Cardiol. 1996. PMID: 8957539 Review.
Cited by
-
Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation.Cardiovasc Res. 2015 Oct 1;108(1):4-20. doi: 10.1093/cvr/cvv205. Epub 2015 Aug 3. Cardiovasc Res. 2015. PMID: 26239654 Free PMC article. Review.
-
Pathophysiology of Calcium Mediated Ventricular Arrhythmias and Novel Therapeutic Options with Focus on Gene Therapy.Int J Mol Sci. 2019 Oct 24;20(21):5304. doi: 10.3390/ijms20215304. Int J Mol Sci. 2019. PMID: 31653119 Free PMC article. Review.
-
Effects of genetic transfection on calcium cycling pathways mediated by double-stranded adeno-associated virus in postinfarction remodeling.J Thorac Cardiovasc Surg. 2020 May;159(5):1809-1819.e3. doi: 10.1016/j.jtcvs.2019.08.089. Epub 2019 Sep 30. J Thorac Cardiovasc Surg. 2020. PMID: 31679707 Free PMC article.
-
S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling.J Biol Chem. 2008 Feb 22;283(8):5046-57. doi: 10.1074/jbc.M709231200. Epub 2007 Dec 17. J Biol Chem. 2008. PMID: 18089560 Free PMC article.
-
AAV-mediated gene therapy for heart failure: enhancing contractility and calcium handling.F1000Prime Rep. 2013 Aug 1;5:27. doi: 10.12703/P5-27. eCollection 2013. F1000Prime Rep. 2013. PMID: 23967378 Free PMC article.
References
-
- American Heart Association. 2003. Heart disease and stroke statistics — 2004 update. American Heart Association. Dallas, Texas, USA. http://www.americanheart.org/presenter.jhtml?identifier=1928.
-
- Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc. Res. 1998;37:279–289. - PubMed
-
- Donato R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003;60:540–551. - PubMed
-
- Remppis A, et al. Altered expression of the Ca(2+)-binding protein S100A1 in human cardiomyopathy. . Biochim. Biophys. Acta. 1996; 1313:253–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

