N-type and L-type calcium channels mediate glycinergic synaptic inputs to retinal ganglion cells of tiger salamanders
- PMID: 15579220
- PMCID: PMC2579891
- DOI: 10.1017/S0952523804214055
N-type and L-type calcium channels mediate glycinergic synaptic inputs to retinal ganglion cells of tiger salamanders
Abstract
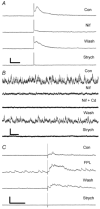
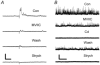
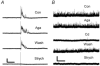
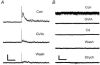
Synaptically localized calcium channels shape the timecourse of synaptic release, are a prominent site for neuromodulation, and have been implicated in genetic disease. In retina, it is well established that L-type calcium channels play a major role in mediating release of glutamate from the photoreceptors and bipolar cells. However, little is known about which calcium channels are coupled to synaptic exocytosis of glycine, which is primarily released by amacrine cells. A recent report indicates that glycine release from spiking AII amacrine cells relies exclusively upon L-type calcium channels. To identify calcium channel types controlling neurotransmitter release from the population of glycinergic neurons that drive retinal ganglion cells, we recorded electrical and potassium evoked inhibitory synaptic currents (IPSCs) from these postsynaptic neurons in retinal slices from tiger salamanders. The L-channel antagonist nifedipine strongly inhibited release and FPL64176, an L-channel agonist, greatly enhanced it, indicating a significant role for L-channels. omega-Conotoxin MVIIC, an N/P/Q-channel antagonist, strongly inhibited release, indicating an important role for non-L channels. While the P/Q-channel blocker omega-Aga IVA produced only small effects, the N-channel blocker omega-conotoxin GVIA strongly inhibited release. Hence, N-type and L-type calcium channels appear to play major roles, overall, in mediating synaptic release of glycine onto retinal ganglion cells.
Figures
References
-
- Bieda MC, Copenhagen DR. Sodium action potentials are not required for light-evoked release of GABA or glycine from retinal amacrine cells. Journal of Neurophysiology. 1999;81:3092–3095. - PubMed
-
- Bieda MC, Copenhagen DR. Inhibition is not required for the production of transient spiking responses from retinal ganglion cells. Visual Neuroscience. 2000;17:243–254. - PubMed
-
- Burgess DL, Noebels JL. Voltage-dependent calcium channel mutations in neurological disease. Annals of the New York Academy of Sciences. 1999;868:199–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

