Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish
- PMID: 15579420
- PMCID: PMC1253666
- DOI: 10.1289/ehp.7209
Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish
Abstract
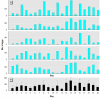
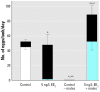
Heightened concern over endocrine-disrupting chemicals is driven by the hypothesis that they could reduce reproductive success and affect wildlife populations, but there is little evidence for this expectation. The pharmaceutical ethynylestradiol (EE2) is a potent endocrine modulator and is present in the aquatic environment at biologically active concentrations. To investigate impacts on reproductive success and mechanisms of disruption, we exposed breeding populations (n = 12) of zebrafish (Danio rerio) over multiple generations to environmentally relevant concentrations of EE2. Life-long exposure to 5 ng/L EE2 in the F1 generation caused a 56% reduction in fecundity and complete population failure with no fertilization. Conversely, the same level of exposure for up to 40 days in mature adults in the parental F0 generation had no impact on reproductive success. Infertility in the F1 generation after life-long exposure to 5 ng/L EE2 was due to disturbed sexual differentiation, with males having no functional testes and either undifferentiated or intersex gonads. These F1 males also showed a reduced vitellogenic response when compared with F0 males, indicating an acclimation to EE2 exposure. Depuration studies found only a partial recovery in reproductive capacity after 5 months. Significantly, even though the F1 males lacked functional testes, they showed male-pattern reproductive behavior, inducing the spawning act and competing with healthy males to disrupt fertilization. Endocrine disruption is therefore likely to affect breeding dynamics and reproductive success in group-spawning fish. Our findings raise major concerns about the population-level impacts for wildlife of long-term exposure to low concentrations of estrogenic endocrine disruptors.
Figures
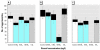
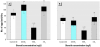

Comment in
-
Roe, interrupted: estrogen exposure impairs fish fertility.Environ Health Perspect. 2004 Dec;112(17):A1010-1. doi: 10.1289/ehp.112-a1010b. Environ Health Perspect. 2004. PMID: 15595175 Free PMC article. No abstract available.
References
-
- Aherne GW, Briggs R. The relevance of the presence of certain synthetic steroids in the aquatic environment. J Pharm Pharmacol. 1989;41:735–736. - PubMed
-
- Andersen L, Holbech H, Gessbo A, Norrgren L, Petersen GI. Effects of exposure to 17α-ethinylestradiol during early development on sexual differentiation and induction of vitellogenin in zebrafish (Danio rerio) Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:365–374. - PubMed
-
- Balch GC, Mackenzie CA, Metcalfe CD. Alterations to gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17α-ethinylestradiol. Environ Toxicol Chem. 2004;23:782–791. - PubMed
-
- Bern HA. 1992. The fragile fetus. In: Chemically-Induced Alterations in Sexual and Functional Development: The Wildlife Human Connection (Colborn T, Clement C, eds). Princeton, NJ:Princeton Scientific Publishing, 9–15.
-
- Bortone SA, Davis WP. Fish intersexuality as indicator of environmental stress. Bioscience. 1994;44:165–172.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

