Hypoxia in renal disease with proteinuria and/or glomerular hypertension
- PMID: 15579441
- PMCID: PMC1618699
- DOI: 10.1016/S0002-9440(10)63249-X
Hypoxia in renal disease with proteinuria and/or glomerular hypertension
Abstract
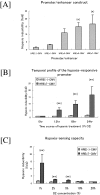
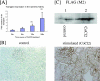
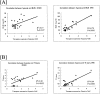
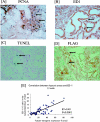
Despite the increasing need to identify and quantify tissue oxygenation at the cellular level, relatively few methods have been available. In this study, we developed a new hypoxia-responsive reporter vector using a hypoxia-responsive element of the 5' vascular endothelial growth factor untranslated region and generated a novel hypoxia-sensing transgenic rat. We then applied this animal model to the detection of tubulointerstitial hypoxia in the diseased kidney. With this model, we were able to identify diffuse cortical hypoxia in the puromycin aminonucleoside-induced nephrotic syndrome and focal and segmental hypoxia in the remnant kidney model. Expression of the hypoxia-responsive transgene increased throughout the observation period, reaching 2.2-fold at 2 weeks in the puromycin aminonucleoside model and 2.6-fold at 4 weeks in the remnant kidney model, whereas that of vascular endothelial growth factor showed a mild decrease, reflecting distinct behaviors of the two genes. The degree of hypoxia showed a positive correlation with microscopic tubulointerstitial injury in both models. Finally, we identified the localization of proliferating cell nuclear antigen-positive, ED-1-positive, and terminal dUTP nick-end labeled-positive cells in the hypoxic cortical area in the remnant kidney model. We propose here a possible pathological tie between chronic tubulointerstitial hypoxia and progressive glomerular diseases.
Figures






References
-
- Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225:485–488. - PubMed
-
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. - PubMed
-
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. - PubMed
-
- Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203:1253–1263. - PubMed
-
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

