Differences in scrapie-induced pathology of the retina and brain in transgenic mice that express hamster prion protein in neurons, astrocytes, or multiple cell types
- PMID: 15579448
- PMCID: PMC1618708
- DOI: 10.1016/S0002-9440(10)63256-7
Differences in scrapie-induced pathology of the retina and brain in transgenic mice that express hamster prion protein in neurons, astrocytes, or multiple cell types
Abstract
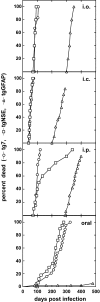
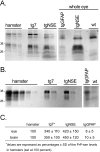
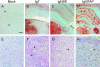
Prion protein (PrP) is expressed in many tissues and is required for susceptibility to scrapie and other prion diseases. To investigate the role of PrP expression in different cell types on pathology in retina and brain after scrapie infection, we examined transgenic mice expressing hamster PrP from the PrP promoter (tg7), the neuron-specific enolase promoter (tgNSE), or the astrocyte-specific glial fibrillary acidic protein promoter (tgGFAP). After intraocular inoculation with hamster scrapie, clinical disease developed in tg7 and tgNSE mice by 100 days and in tgGFAP mice by 350 days. Astrogliosis and scrapie-associated protease-resistant PrP (PrP-res) were detected in retina and brain before clinical onset. Retinal PrP-res was present in high amounts in both tg7 and tgNSE mice, however only tg7 mice developed retinal degeneration and extensive apoptosis. In contrast, in all three lines of mice high levels of brain PrP-res accompanied by neurodegeneration were observed. Thus, PrP expression on neurons or astrocytes was sufficient for development of scrapie-induced degeneration in brain but not in retina. The combined effects of PrP-res production in multiple cell types was required to produce retinal degeneration, whereas in brain PrP-res production by neurons or astrocytes alone was sufficient to cause neuronal damage via direct or indirect mechanisms.
Figures



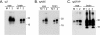
Similar articles
-
Scrapie-specific neuronal lesions are independent of neuronal PrP expression.Ann Neurol. 2004 Jun;55(6):781-92. doi: 10.1002/ana.20093. Ann Neurol. 2004. PMID: 15174012
-
Prion protein expression differences in microglia and astroglia influence scrapie-induced neurodegeneration in the retina and brain of transgenic mice.J Virol. 2007 Oct;81(19):10340-51. doi: 10.1128/JVI.00865-07. Epub 2007 Jul 25. J Virol. 2007. PMID: 17652390 Free PMC article.
-
Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death.Proc Natl Acad Sci U S A. 2004 Aug 17;101(33):12271-6. doi: 10.1073/pnas.0402725101. Epub 2004 Aug 9. Proc Natl Acad Sci U S A. 2004. PMID: 15302929 Free PMC article.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
-
The neuropathological phenotype in transgenic mice expressing different prion protein constructs.Philos Trans R Soc Lond B Biol Sci. 1994 Mar 29;343(1306):415-23. doi: 10.1098/rstb.1994.0038. Philos Trans R Soc Lond B Biol Sci. 1994. PMID: 7913760 Review.
Cited by
-
Lower specific infectivity of protease-resistant prion protein generated in cell-free reactions.Proc Natl Acad Sci U S A. 2011 Nov 29;108(48):E1244-53. doi: 10.1073/pnas.1111255108. Epub 2011 Nov 7. Proc Natl Acad Sci U S A. 2011. PMID: 22065744 Free PMC article.
-
Uptake and degradation of protease-sensitive and -resistant forms of abnormal human prion protein aggregates by human astrocytes.Am J Pathol. 2014 Dec;184(12):3299-307. doi: 10.1016/j.ajpath.2014.08.005. Epub 2014 Sep 30. Am J Pathol. 2014. PMID: 25280631 Free PMC article.
-
The role of astrocytes in prion-like mechanisms of neurodegeneration.Brain. 2022 Mar 29;145(1):17-26. doi: 10.1093/brain/awab366. Brain. 2022. PMID: 35265969 Free PMC article. Review.
-
Effect of host and strain factors on α-synuclein prion pathogenesis.Trends Neurosci. 2024 Jul;47(7):538-550. doi: 10.1016/j.tins.2024.05.004. Epub 2024 May 27. Trends Neurosci. 2024. PMID: 38806297 Free PMC article. Review.
-
Identification of a novel endoplasmic reticulum stress response element regulated by XBP1.J Biol Chem. 2013 Jul 12;288(28):20378-91. doi: 10.1074/jbc.M113.457242. Epub 2013 Jun 4. J Biol Chem. 2013. PMID: 23737521 Free PMC article.
References
-
- Chesebro B, Race R, Wehrly K, Nishio J, Bloom M, Lechner D, Bergstrom S, Robbins K, Mayer L, Keith JM. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985;315:331–333. - PubMed
-
- Oesch B, Westaway D, Walchli M, McKinley MP, Kent SB, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985;40:735–746. - PubMed
-
- Basler K, Oesch B, Scott M, Westaway D, Walchli M, Groth DF, McKinley MP, Prusiner SB, Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986;46:417–428. - PubMed
-
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. - PubMed
-
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

