T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis
- PMID: 15579449
- PMCID: PMC1618702
- DOI: 10.1016/S0002-9440(10)63257-9
T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis
Abstract
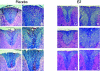
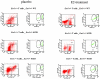
Gender influences mediated by 17 beta-estradiol (E2) have been associated with susceptibility to and severity of autoimmune diseases such as diabetes, arthritis, and multiple sclerosis. In this regard, we have shown that estrogen receptor-alpha (Esr1) is crucial for the protective effect of 17 beta-estradiol (E2) in murine experimental autoimmune encephalitis (EAE), an animal model of multiple sclerosis. The expression of estrogen receptors among various immune cells (eg, T and B lymphocytes, antigen-presenting cells) suggests that the therapeutic effect of E2 is likely mediated directly through specific receptor binding. However, the target immune cell populations responsive to E2 treatment have not been identified. In the current study, we induced EAE in T-cell-deficient, severe combined immunodeficient mice or in immunocompetent mice with encephalitogenic T cells from wild-type Esr1+/+ or Esr1 knockout (Esr1-/-) donors and compared the protective E2 responses. The results showed that E2-responsive, Esr1+/+ disease-inducing encephalitogenic T cells were neither necessary nor sufficient for E2-mediated protection from EAE. Instead, the therapeutic response appeared to be mediated through direct effects on nonlymphocytic, E2-responsive cells and down-regulation of the inflammatory response in the central nervous system. These results provide the first demonstration that the protective effect of E2 on EAE is not mediated directly through E2-responsive T cells and raise the alternative possibility that nonlymphocytic cells such as macrophages, dendritic cells, or other nonlymphocytic cells are primarily responsive to E2 treatment in EAE.
Figures


Similar articles
-
Estrogen receptor α signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis.J Immunol. 2011 Sep 1;187(5):2386-93. doi: 10.4049/jimmunol.1101578. Epub 2011 Aug 1. J Immunol. 2011. PMID: 21810607
-
The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha.Am J Pathol. 2003 Oct;163(4):1599-605. doi: 10.1016/s0002-9440(10)63516-x. Am J Pathol. 2003. PMID: 14507666 Free PMC article.
-
Neuroimmunoprotective effects of estrogen and derivatives in experimental autoimmune encephalomyelitis: therapeutic implications for multiple sclerosis.J Neurosci Res. 2004 Dec 1;78(5):603-24. doi: 10.1002/jnr.20330. J Neurosci Res. 2004. PMID: 15515048 Review.
-
Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis.J Immunol. 2004 Aug 15;173(4):2435-42. doi: 10.4049/jimmunol.173.4.2435. J Immunol. 2004. PMID: 15294957
-
A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis.Ann N Y Acad Sci. 2006 Nov;1089:343-72. doi: 10.1196/annals.1386.021. Ann N Y Acad Sci. 2006. PMID: 17261780 Review.
Cited by
-
Gender and sex hormones in multiple sclerosis pathology and therapy.Front Biosci (Landmark Ed). 2009 Jan 1;14(12):4477-515. doi: 10.2741/3543. Front Biosci (Landmark Ed). 2009. PMID: 19273365 Free PMC article. Review.
-
Autoimmunity in Coxsackievirus B3 induced myocarditis: role of estrogen in suppressing autoimmunity.Future Virol. 2010 May 1;5(3):273-286. doi: 10.2217/fvl.10.19. Future Virol. 2010. PMID: 20963181 Free PMC article.
-
PD-L1 is required for estrogen-induced protection against severe EAE in IL-10 deficient mice1.Metab Brain Dis. 2023 Feb;38(2):589-599. doi: 10.1007/s11011-022-01129-8. Epub 2022 Dec 1. Metab Brain Dis. 2023. PMID: 36454506 Free PMC article.
-
PD-1, gender, and autoimmunity.Autoimmun Rev. 2010 Jun;9(8):583-7. doi: 10.1016/j.autrev.2010.04.003. Epub 2010 Apr 28. Autoimmun Rev. 2010. PMID: 20433954 Free PMC article. Review.
-
Tissue-Dependent Expression of Estrogen Receptor β in 17β-Estradiol-Mediated Attenuation of Autoimmune CNS Inflammation.Open Autoimmun J. 2010 Jan 1;2:197-204. doi: 10.2174/1876894601002010197. Open Autoimmun J. 2010. PMID: 22242109 Free PMC article.
References
-
- Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1280. - PubMed
-
- Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res. 1998;47:290–301. - PubMed
-
- Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96:457–462. - PubMed
-
- Birk K, Ford C, Smeltzer S, Ryan D, Miller R, Rudick RA. The clinical course of multiple sclerosis during pregnancy and the puerperium. Arch Neurol. 1990;47:738–742. - PubMed
-
- Korn-Lubetzki I, Kahana E, Cooper G, Abramsky O. Activity of multiple sclerosis during pregnancy and puerperium. Ann Neurol. 1984;16:229–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

