Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells
- PMID: 15579540
- PMCID: PMC1665368
- DOI: 10.1113/jphysiol.2004.075168
Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells
Abstract
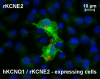
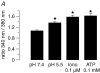
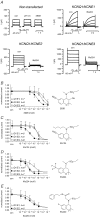
Recently, we and others have shown that luminal K+ recycling via KCNQ1 K+ channels is required for gastric H+ secretion. Inhibition of KCNQ1 by the chromanol 293B strongly diminished H+ secretion. The present study aims at clarifying KCNQ1 subunit composition, subcellular localization, regulation and pharmacology in parietal cells. Using in situ hybridization and immunofluorescence techniques, we identified KCNE2 as the beta subunit of KCNQ1 in the luminal membrane compartment of parietal cells. Expressed in COS cells, hKCNE2/hKCNQ1 channels were activated by acidic pH, PIP2, cAMP and purinergic receptor stimulation. Qualitatively similar results were obtained in mouse parietal cells. Confocal microscopy revealed stimulation-induced translocation of H+,K+-ATPase from tubulovesicles towards the luminal pole of parietal cells, whereas distribution of KCNQ1 K+ channels did not change to the same extent. In COS cells the 293B-related substance IKs124 blocked hKCNE2/hKCNQ1 with an IC50 of 8 nM. Inhibition of hKCNE1- and hKCNE3-containing channels was weaker with IC50 values of 370 and 440 nM, respectively. In conclusion, KCNQ1 coassembles with KCNE2 to form acid-activated luminal K+ channels of parietal cells. KCNQ1/KCNE2 is activated during acid secretion via several pathways but probably not by targeting of the channel to the membrane. IKs124 could serve as a leading compound in the development of subunit-specific KCNE2/KCNQ1 blockers to treat peptic ulcers.
Figures


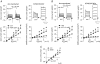



References
-
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- Aihara T, Nakamura E, Amagase K, Tomita K, Fujishita T, Furutani K, Okabe S. Pharmacol Ther. 2003;98:109–127. - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Chow DC, Forte JG. Characterization of the beta-subunit of the H+-K+-ATPase using an inhibitory monoclonal antibody. Am J Physiol. 1993;265:C1562–C1570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

