The role of the cell cycle machinery in resumption of postembryonic development
- PMID: 15579664
- PMCID: PMC548844
- DOI: 10.1104/pp.104.049361
The role of the cell cycle machinery in resumption of postembryonic development
Abstract
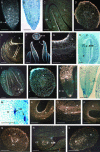
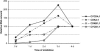
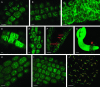
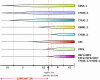
Cell cycle activity is required for plant growth and development, but its involvement in the early events that initiate seedling development remains to be clarified. We performed experiments aimed at understanding when cell cycle progression is activated during seed germination, and what its contribution is for proper seedling establishment. To this end, the spatial and temporal expression profiles of a large set of cell cycle control genes in germinating seeds of Arabidopsis (Arabidopsis thaliana) and white cabbage (Brassica oleracea) were analyzed. The in vivo behavior of the microtubular cytoskeleton was monitored during Arabidopsis seed germination. Flow cytometry of Arabidopsis germinating seeds indicated that DNA replication was mainly initiated at the onset of root protrusion, when germination reached its end. Expression analysis of cell cycle genes with mRNA in situ localization, beta-glucuronidase assays, and semiquantitative reverse transcription-polymerase chain reaction showed that transcription of most cell cycle genes was detected only after completion of germination. In vivo green fluorescent protein analysis of the microtubule cytoskeleton demonstrated that mitosis-specific microtubule arrays occurred only when the radicle had started to protrude, although the assembly of the microtubular cytoskeleton was promptly activated once germination was initiated. Thus, seed germination involves the synthesis and/or activation of a reduced number of core cell cycle proteins, which only trigger DNA replication, but is not sufficient to drive cells into mitosis. Mitotic divisions are observed only after the radicle has protruded and presumably rely on the de novo production of other cell cycle regulators.
Figures


References
-
- Azimzadeh J, Traas J, Pastuglia M (2001) Molecular aspects of microtubule dynamics in plants. Curr Opin Plant Biol 4: 513–519 - PubMed
-
- Burssens S, de Almeida Engler J, Beeckman T, Richard C, Shaul O, Ferreira P, Van Montagu M, Inzé D (2000) Developmental expression of the Arabidopsis thaliana CycA2;1 gene. Planta 211: 623–631 - PubMed
-
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11: 113–116
-
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

