Host factors that affect Ty3 retrotransposition in Saccharomyces cerevisiae
- PMID: 15579677
- PMCID: PMC1448793
- DOI: 10.1534/genetics.104.028126
Host factors that affect Ty3 retrotransposition in Saccharomyces cerevisiae
Abstract
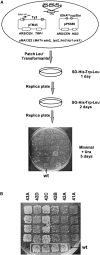
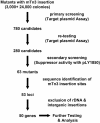
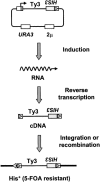
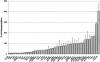
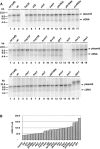
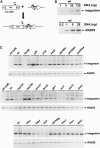
The retrovirus-like element Ty3 of Saccharomyces cerevisiae integrates at the transcription initiation region of RNA polymerase III. To identify host genes that affect transposition, a collection of insertion mutants was screened using a genetic assay in which insertion of Ty3 activates expression of a tRNA suppressor. Fifty-three loci were identified in this screen. Corresponding knockout mutants were tested for the ability to mobilize a galactose-inducible Ty3, marked with the HIS3 gene. Of 42 mutants tested, 22 had phenotypes similar to those displayed in the original assay. The proteins encoded by the defective genes are involved in chromatin dynamics, transcription, RNA processing, protein modification, cell cycle regulation, nuclear import, and unknown functions. These mutants were induced for Ty3 expression and assayed for Gag3p protein, integrase, cDNA, and Ty3 integration upstream of chromosomal tDNA(Val(AAC)) genes. Most mutants displayed differences from the wild type in one or more intermediates, although these were typically not as severe as the genetic defect. Because a relatively large number of genes affecting retrotransposition can be identified in yeast and because the majority of these genes have mammalian homologs, this approach provides an avenue for the identification of potential antiviral targets.
Figures


References
-
- Allen, N. P., L. Huang, A. Burlingame and M. Rexach, 2001. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 276: 29268–29274. - PubMed
-
- Aye, M., and S. B. Sandmeyer, 2003. Ty3 requires yeast La homologous protein for wild-type frequencies of transposition. Mol. Microbiol. 49: 501–515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

