Gene interactions in Caenorhabditis elegans define DPY-31 as a candidate procollagen C-proteinase and SQT-3/ROL-4 as its predicted major target
- PMID: 15579684
- PMCID: PMC1448789
- DOI: 10.1534/genetics.104.027953
Gene interactions in Caenorhabditis elegans define DPY-31 as a candidate procollagen C-proteinase and SQT-3/ROL-4 as its predicted major target
Abstract
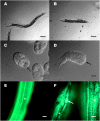
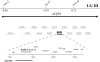
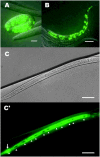
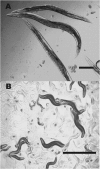
Zinc metalloproteases of the BMP-1/TOLLOID family (also known as astacins) are extracellular enzymes involved in important developmental processes in metazoans. We report the characterization of the Caenorhabditis elegans gene dpy-31, which encodes the first essential astacin metalloprotease identified in this organism. Loss-of-function mutations in dpy-31 result in cuticle defects, abnormal morphology, and embryonic lethality, indicating that dpy-31 is required for formation of the collagenous exoskeleton. DPY-31 is widely expressed in the hypodermal cells, which are responsible for cuticle secretion. We have investigated the dpy-31 function through reversion analysis. While complete reversion can be obtained only by intragenic suppressors, reversion of the Dpy-31 lethal phenotype also can be caused by dominant extragenic suppressors. Nine extragenic suppressors carry mutations in the uniquely essential collagen gene sqt-3, which we show is the same gene as rol-4. Most mutations exhibit the unusual property of exclusively dominant suppression and all affect the sequence of the SQT-3 collagen C terminus. This suggests that DPY-31 is responsible for C-terminal proteolytic processing of collagen trimers and is therefore a structural and functional homolog of vertebrate BMP-1. The results also demonstrate the critical importance of the collagen C-terminal sequence, which is highly conserved among all 49 members of the SQT-3 subfamily.
Figures



Similar articles
-
The C terminus of collagen SQT-3 has complex and essential functions in nematode collagen assembly.Genetics. 2006 Apr;172(4):2253-67. doi: 10.1534/genetics.105.053637. Epub 2006 Feb 1. Genetics. 2006. PMID: 16452136 Free PMC article.
-
Collagen processing and cuticle formation is catalysed by the astacin metalloprotease DPY-31 in free-living and parasitic nematodes.Int J Parasitol. 2010 Apr;40(5):533-42. doi: 10.1016/j.ijpara.2009.10.007. Epub 2009 Oct 31. Int J Parasitol. 2010. PMID: 19883650
-
Caenorhabditis elegans dpy-5 is a cuticle procollagen processed by a proprotein convertase.Cell Mol Life Sci. 2006 May;63(10):1193-204. doi: 10.1007/s00018-006-6012-z. Cell Mol Life Sci. 2006. PMID: 16649143 Free PMC article.
-
Developmental roles of the BMP1/TLD metalloproteinases.Birth Defects Res C Embryo Today. 2006 Mar;78(1):47-68. doi: 10.1002/bdrc.20060. Birth Defects Res C Embryo Today. 2006. PMID: 16622848 Review.
-
Meprins, membrane-bound and secreted astacin metalloproteinases.Mol Aspects Med. 2008 Oct;29(5):309-28. doi: 10.1016/j.mam.2008.08.002. Epub 2008 Aug 22. Mol Aspects Med. 2008. PMID: 18783725 Free PMC article. Review.
Cited by
-
The Collagens DPY-17 and SQT-3 Direct Anterior-Posterior Migration of the Q Neuroblasts in C. elegans.J Dev Biol. 2021 Feb 19;9(1):7. doi: 10.3390/jdb9010007. J Dev Biol. 2021. PMID: 33669899 Free PMC article.
-
Game of Tissues: How the Epidermis Thrones C. elegans Shape.J Dev Biol. 2020 Mar 9;8(1):7. doi: 10.3390/jdb8010007. J Dev Biol. 2020. PMID: 32182901 Free PMC article. Review.
-
The C terminus of collagen SQT-3 has complex and essential functions in nematode collagen assembly.Genetics. 2006 Apr;172(4):2253-67. doi: 10.1534/genetics.105.053637. Epub 2006 Feb 1. Genetics. 2006. PMID: 16452136 Free PMC article.
-
A highly conserved, inhibitable astacin metalloprotease from Teladorsagia circumcincta is required for cuticle formation and nematode development.Int J Parasitol. 2015 Apr;45(5):345-55. doi: 10.1016/j.ijpara.2015.01.004. Epub 2015 Feb 28. Int J Parasitol. 2015. PMID: 25736599 Free PMC article.
-
Molecular Characterization and Virus-Induced Gene Silencing of a Collagen Gene, Me-col-1, in Root-Knot Nematode Meloidogyne enterolobii.Life (Basel). 2022 Dec 14;12(12):2103. doi: 10.3390/life12122103. Life (Basel). 2022. PMID: 36556467 Free PMC article.
References
-
- Ahmed, S., and J. Hodgkin, 2000. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature 403: 159–164. - PubMed
-
- Bachinger, H. P., P. Bruckner, R. Timpl, D. J. Prockop and J. Engel, 1980. Folding mechanism of the triple helix in type-III collagen and type-III pN-collagen. Role of disulfide bridges and peptide bond isomerization. Eur. J. Biochem. 106: 619–632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

