beta-Peptides as inhibitors of protein-protein interactions
- PMID: 15582447
- PMCID: PMC2853017
- DOI: 10.1016/j.bmc.2004.09.009
beta-Peptides as inhibitors of protein-protein interactions
Abstract
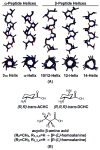
We became interested several years ago in exploring whether 14-helical beta-peptide foldamers could bind protein surfaces and inhibit protein-protein interactions, and if so, whether their affinities and specificities would compare favorably with those of natural or miniature proteins. This exploration was complicated initially by the absence of a suitable beta-peptide scaffold, one that possessed a well-defined 14-helical structure in water and tolerated the diverse sequence variation required to generate high-affinity protein surface ligands. In this perspective, we describe our approach to the design of adaptable beta-peptide scaffolds with high levels of 14-helix structure in water, track the subsequent development of 14-helical beta-peptide protein-protein interaction inhibitors, and examine the potential of this strategy for targeting other therapeutically important proteins.
Figures

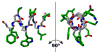


References
-
- Schepartz A, Kim PS. Interaction, assembly and processing—at the chemistry–biology interface—overview. Curr Opin Chem Biol. 1998;2:9. - PubMed
-
- Marshall SA, Lazar GA, Chirino AJ, Desjarlais JR. Rational design and engineering of therapeutic proteins. Drug Discov Today. 2003;8:212. - PubMed
-
- Gellman SH. Foldamers: A manifesto. Accounts Chem Res. 1998;31:173.
-
- Hill JH, Mio MJ, Prince RB, Hughes TS, Moore JS. A field guide to foldamers. Chem Rev. 2001;101:3893. - PubMed
-
- Frackenpohl J, Arvidsson PI, Schreiber JV, Seebach D. The outstanding biological stability of beta- and gamma-peptides toward proteolytic enzymes: an in vitro investigation with fifteen peptidases. Chembiochem. 2001;2:445. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

