Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory
- PMID: 15583011
- PMCID: PMC2211955
- DOI: 10.1084/jem.20031763
Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory
Abstract
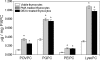
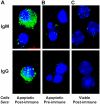
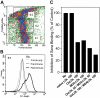
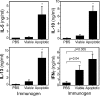
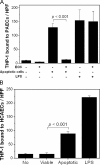
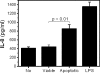
Oxidation of low density lipoprotein (LDL) generates a variety of oxidatively modified lipids and lipid-protein adducts that are immunogenic and proinflammatory, which in turn contribute to atherogenesis. Cells undergoing apoptosis also display oxidized moieties on their surface membranes, as determined by binding of oxidation-specific monoclonal antibodies. In the present paper, we demonstrated by mass spectrometry that in comparison with viable cells, membranes of cells undergoing apoptosis contain increased levels of biologically active oxidized phospholipids (OxPLs). Indeed, immunization of mice with syngeneic apoptotic cells induced high autoantibody titers to various oxidation-specific epitopes of oxidized LDL, including OxPLs containing phosphorylcholine, whereas immunization with viable thymocytes, primary necrotic thymocytes, or phosphate-buffered saline did not. Reciprocally, these antisera specifically bound to apoptotic cells through the recognition of oxidation-specific epitopes. Moreover, splenocyte cultures from mice immunized with apoptotic cells spontaneously released significant levels of T helper cell (Th) 1 and Th2 cytokines, whereas splenocytes from controls yielded only low levels. Finally, we demonstrated that the OxPLs of apoptotic cells activated endothelial cells to induce monocyte adhesion, a proinflammatory response that was abrogated by an antibody specific to oxidized phosphatidylcholine. These results suggest that apoptotic cell death generates oxidatively modified moieties, which can induce autoimmune responses and a local inflammatory response by recruiting monocytes via monocyte-endothelial cell interaction.
Figures


References
-
- Cocca, B.A., A.M. Cline, and M.Z. Radic. 2002. Blebs and apoptotic bodies are B cell autoantigens. J. Immunol. 169:159–166. - PubMed
-
- Price, B.E., J. Rauch, M.A. Shia, M.T. Walsh, W. Lieberthal, H.M. Gilligan, T. O'Laughlin, J.S. Koh, and J.S. Levine. 1996. Anti-phospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a beta 2-glycoprotein I-dependent manner. J. Immunol. 157:2201–2208. - PubMed
-
- Levine, J.S., J.S. Koh, R. Subang, and J. Rauch. 1999. Apoptotic cells as immunogen and antigen in the antiphospholipid syndrome. Exp. Mol. Pathol. 66:82–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

