Crucial role for ecto-5'-nucleotidase (CD73) in vascular leakage during hypoxia
- PMID: 15583013
- PMCID: PMC1237012
- DOI: 10.1084/jem.20040915
Crucial role for ecto-5'-nucleotidase (CD73) in vascular leakage during hypoxia
Abstract
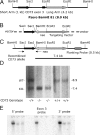
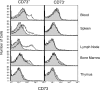
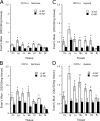
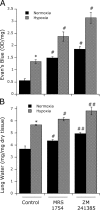
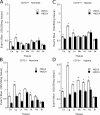
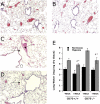
Extracellular adenosine has been widely implicated in adaptive responses to hypoxia. The generation of extracellular adenosine involves phosphohydrolysis of adenine nucleotide intermediates, and is regulated by the terminal enzymatic step catalyzed by ecto-5'-nucleotidase (CD73). Guided by previous work indicating that hypoxia-induced vascular leakage is, at least in part, controlled by adenosine, we generated mice with a targeted disruption of the third coding exon of Cd73 to test the hypothesis that CD73-generated extracellular adenosine functions in an innate protective pathway for hypoxia-induced vascular leakage. Cd73(-/-) mice bred and gained weight normally, and appeared to have an intact immune system. However, vascular leakage was significantly increased in multiple organs, and after subjection to normobaric hypoxia (8% O(2)), Cd73(-/-) mice manifested fulminant vascular leakage, particularly prevalent in the lung. Histological examination of lungs from hypoxic Cd73(-/-) mice revealed perivascular interstitial edema associated with inflammatory infiltrates surrounding larger pulmonary vessels. Vascular leakage secondary to hypoxia was reversed in part by adenosine receptor agonists or reconstitution with soluble 5'-nucleotidase. Together, our studies identify CD73 as a critical mediator of vascular leakage in vivo.
Figures
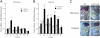

References
-
- Webb, A.R. 2000. Capillary leak. Pathogenesis and treatment. Minerva Anestesiol. 66:255–263. - PubMed
-
- Stevens, T., J.G.N. Garcia, D.M. Shasby, J. Bhattacharya, and A.B. Malik. 2000. Mechanisms regulating endothelial cell barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L419–L422. - PubMed
-
- Michel, C.C., and F.E. Curry. 1999. Microvascular permeability. Physiol. Rev. 79:703–761. - PubMed
-
- Rippe, B., and B. Haraldsson. 1994. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol. Rev. 74:163–219. - PubMed
-
- Lum, H., and A.B. Malik. 1994. Regulation of vascular endothelial barrier function. Am. J. Physiol. 267:L223–L241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

