A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation
- PMID: 15583026
- PMCID: PMC2172467
- DOI: 10.1083/jcb.200410090
A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation
Abstract
Millions of patients suffer from Alzheimer's disease, and intensive efforts to find a cure for this devastating disorder center on the proteases, which release the deadly amyloid beta-peptide from its precursor. The cutting procedure is thought to be cholesterol dependent and strategies to lower cholesterol as therapeutic treatment are under intensive investigation. Recent findings suggest that the complete proteolytic machinery required for amyloid beta-peptide generation is located within lipid rafts. Data by Dotti and colleagues (Abad-Rodriguez et al., 2004), in this issue, suggest that rafts isolate the cutting machinery away from its deadly substrate. These findings describe a novel mechanism for controlling proteolytic activity by building a lipid boundary between proteases and their substrates.
Figures
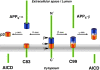

Comment on
-
Neuronal membrane cholesterol loss enhances amyloid peptide generation.J Cell Biol. 2004 Dec 6;167(5):953-60. doi: 10.1083/jcb.200404149. J Cell Biol. 2004. PMID: 15583033 Free PMC article.
References
-
- Corder, E.H., A.M. Saunders, W.J. Strittmatter, D.E. Schmechel, P.C. Gaskell, G.W. Small, A.D. Roses, J.L. Haines, and M.A. Pericak-Vance. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 261:921–923. - PubMed
-
- Fassbender, K., M. Simons, C. Bergmann, M. Stroick, D. Lutjohann, P. Keller, H. Runz, S. Kuhl, T. Bertsch, K. von Bergmann, et al. 2001. Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 98:5856–5861. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

