Neuronal membrane cholesterol loss enhances amyloid peptide generation
- PMID: 15583033
- PMCID: PMC2172459
- DOI: 10.1083/jcb.200404149
Neuronal membrane cholesterol loss enhances amyloid peptide generation
Abstract
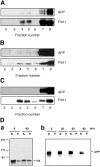
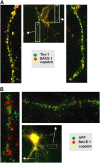
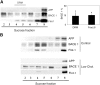
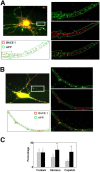
Recent experimental and clinical retrospective studies support the view that reduction of brain cholesterol protects against Alzheimer's disease (AD). However, genetic and pharmacological evidence indicates that low brain cholesterol leads to neurodegeneration. This apparent contradiction prompted us to analyze the role of neuronal cholesterol in amyloid peptide generation in experimental systems that closely resemble physiological and pathological situations. We show that, in the hippocampus of control human and transgenic mice, only a small pool of endogenous APP and its beta-secretase, BACE 1, are found in the same membrane environment. Much higher levels of BACE 1-APP colocalization is found in hippocampal membranes from AD patients or in rodent hippocampal neurons with a moderate reduction of membrane cholesterol. Their increased colocalization is associated with elevated production of amyloid peptide. These results suggest that loss of neuronal membrane cholesterol contributes to excessive amyloidogenesis in AD and pave the way for the identification of the cause of cholesterol loss and for the development of specific therapeutic strategies.
Figures

Comment in
-
A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation.J Cell Biol. 2004 Dec 6;167(5):809-12. doi: 10.1083/jcb.200410090. J Cell Biol. 2004. PMID: 15583026 Free PMC article. Review.
References
-
- Austen, B., G. Christodoulou, and J.E. Terry. 2002. Relation between cholesterol levels, statins and Alzheimer's disease in the human population. J. Nutr. Health Aging. 6:377–382. - PubMed
-
- Burns, M., and K. Duff. 2002. Cholesterol in Alzheimer's disease and tauopathy. Ann. N. Y. Acad. Sci. 977:367–375. - PubMed
-
- Corder, E.H., A.M. Saunders, W.J. Strittmatter, D.E. Schmechel, P.C. Gaskell, G.W. Small, A.D. Roses, J.L. Haines, and M.A. Pericak-Vance. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 261:921–923. - PubMed
-
- Cucchiara, B., and S.E. Kasner. 2001. Use of statins in CNS disorders. J. Neurol. Sci. 187:81–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

