Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts
- PMID: 15583034
- PMCID: PMC2172447
- DOI: 10.1083/jcb.200409077
Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts
Abstract
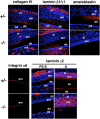
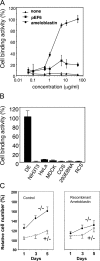
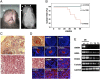
Tooth morphogenesis results from reciprocal interactions between oral epithelium and ectomesenchyme culminating in the formation of mineralized tissues, enamel, and dentin. During this process, epithelial cells differentiate into enamel-secreting ameloblasts. Ameloblastin, an enamel matrix protein, is expressed by differentiating ameloblasts. Here, we report the creation of ameloblastin-null mice, which developed severe enamel hypoplasia. In mutant tooth, the dental epithelium differentiated into enamel-secreting ameloblasts, but the cells were detached from the matrix and subsequently lost cell polarity, resumed proliferation, and formed multicell layers. Expression of Msx2, p27, and p75 were deregulated in mutant ameloblasts, the phenotypes of which were reversed to undifferentiated epithelium. We found that recombinant ameloblastin adhered specifically to ameloblasts and inhibited cell proliferation. The mutant mice developed an odontogenic tumor of dental epithelium origin. Thus, ameloblastin is a cell adhesion molecule essential for amelogenesis, and it plays a role in maintaining the differentiation state of secretory stage ameloblasts by binding to ameloblasts and inhibiting proliferation.
Figures





References
-
- Abiko, Y., M. Murata, Y. Ito, T. Taira, M. Nishimura, M. Arisue, T. Inoue, M. Shimono, Y. Kuboki, and T. Kaku. 2001. Immunohistochemical localization of amelogenin in human odontogenic tumors, using a polyclonal antibody against bovine amelogenin. Med. Electron Microsc. 34:185–189. - PubMed
-
- Adams, J.C., and F.M. Watt. 1993. Regulation of development and differentiation by the extracellular matrix. Development. 117:1183–1198. - PubMed
-
- Bloch-Zupan, A., T. Leveillard, P. Gorry, J.L. Fausser, and J.V. Ruch. 1998. Expression of p21(WAF1/CIP1) during mouse odontogenesis. Eur. J. Oral Sci. 106(Suppl 1):104–111. - PubMed
-
- Bosshardt, D.D., and A. Nanci. 1998. Immunolocalization of epithelial and mesenchymal matrix constituents in association with inner enamel epithelial cells. J. Histochem. Cytochem. 46:135–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

