Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor
- PMID: 15585581
- PMCID: PMC535104
- DOI: 10.1073/pnas.0408030101
Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor
Abstract
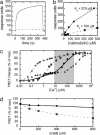
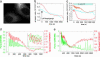
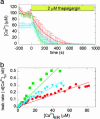
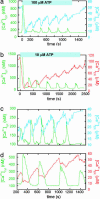
The endoplasmic reticulum (ER) serves as a cellular storehouse for Ca(2+), and Ca(2+) released from the ER plays a role in a host of critical signaling reactions, including exocytosis, contraction, metabolism, regulation of transcription, fertilization, and apoptosis. Given the central role played by the ER, our understanding of these signaling processes could be greatly enhanced by the ability to image [Ca(2+)](ER) directly in individual cells. We created a genetically encoded Ca(2+) indicator by redesigning the binding interface of calmodulin and a calmodulin-binding peptide. The sensor has improved reaction kinetics and a K(d) ideal for imaging Ca(2+) in the ER and is no longer perturbed by large excesses of native calmodulin. Importantly, it provides a significant improvement over all previous methods for monitoring [Ca(2+)](ER) and has been used to directly show that, in MCF-7 breast cancer cells, the antiapoptotic protein B cell lymphoma 2 (Bcl-2) (i) lowers [Ca(2+)](ER) by increasing Ca(2+) leakage under resting conditions and (ii) alters Ca(2+) oscillations induced by ATP, and that acute inhibition of Bcl-2 by the green tea compound epigallocatechin gallate results in an increase in [Ca(2+)](ER) due to inhibition of Bcl-2-mediated Ca(2+) leakage.
Figures

References
-
- Alvarez, J. & Montero, M. (2002) Cell Calcium 32, 251–260. - PubMed
-
- Solovyova, N. & Verkhratsky, A. (2002) J. Neurosci. Methods 122, 1–12. - PubMed
-
- Arnaudeau, S., Kelley, W. L., Walsh, J. V., Jr., & Demaurex, N. (2001) J. Biol. Chem. 276, 29430–29439. - PubMed
-
- Filippin, L., Magalhaes, P. J., Benedetto, G. D., Colella, M. & Pozzan, T. (2003) J. Biol. Chem. 278, 39224–39234. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

