Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans
- PMID: 15585584
- PMCID: PMC535103
- DOI: 10.1073/pnas.0403504101
Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans
Abstract
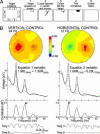
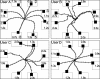
Brain-computer interfaces (BCIs) can provide communication and control to people who are totally paralyzed. BCIs can use noninvasive or invasive methods for recording the brain signals that convey the user's commands. Whereas noninvasive BCIs are already in use for simple applications, it has been widely assumed that only invasive BCIs, which use electrodes implanted in the brain, can provide multidimensional movement control of a robotic arm or a neuroprosthesis. We now show that a noninvasive BCI that uses scalp-recorded electroencephalographic activity and an adaptive algorithm can provide humans, including people with spinal cord injuries, with multidimensional point-to-point movement control that falls within the range of that reported with invasive methods in monkeys. In movement time, precision, and accuracy, the results are comparable to those with invasive BCIs. The adaptive algorithm used in this noninvasive BCI identifies and focuses on the electroencephalographic features that the person is best able to control and encourages further improvement in that control. The results suggest that people with severe motor disabilities could use brain signals to operate a robotic arm or a neuroprosthesis without needing to have electrodes implanted in their brains.
Figures


References
-
- Wolpaw, J. R., Birbaumer, N., McFarland, D. J., Pfurtscheller, G. & Vaughan, T. M. (2002) Clin. Neurophysiol. 113, 767-791. - PubMed
-
- Birbaumer, N., Ghanayim, N., Hinterberger, T., Iversen, I., Kotchoubey, B., Kübler, A., Perelmouter, J., Taub, E. & Flor, H. (1999) Nature 398, 297-298. - PubMed
-
- Pfurtscheller, G., Neuper, C., Müller, G. R., Obermaier, B., Krausz, G., Schlögl, A., Scherer, R., Graimann, B., Keinrath, C., Skliris, D., et al. (2003) IEEE Trans. Neural Syst. Rehabil. Eng. 11, 177-180. - PubMed
-
- Chapin, J. K., Moxon, K. A., Markowitz, R. S. & Nicolelis, M. A. L. (1999) Nat. Neurosci. 2, 664-670. - PubMed
-
- Wessberg, J., Stambaugh, C. R., Kralik, J., Beck, P. D., Laubach, M., Chapin, J. K., Kim, J., Biggs, J., Srinivasan, M. A. & Nicolelis, M. A. (2000) Nature 408, 361-365. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

