Basic helix-loop-helix transcription factor Tcfl5 interacts with the Calmegin gene promoter in mouse spermatogenesis
- PMID: 15585666
- PMCID: PMC535687
- DOI: 10.1093/nar/gkh979
Basic helix-loop-helix transcription factor Tcfl5 interacts with the Calmegin gene promoter in mouse spermatogenesis
Abstract
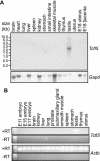
In mouse spermatogenesis, differentiating germ line cells initiate expression of specific genes at subsequent developmental steps. The Calmegin (Clgn) gene is first expressed in meiotic prophase, in primary spermatocytes, and encodes a protein that acts as a chaperone. To identify testis-specific transcription factors that control expression of the Clgn gene in spermatogenesis, we performed a yeast one-hybrid screening with a Clgn promoter sequence as bait DNA. This screening resulted in the identification of mouse Tcfl5 as a candidate Clgn promoter-binding protein. Tcfl5 is a member of the basic helix-loop-helix (bHLH) family of transcription factors, and mouse Tcfl5 shows 83% amino acid sequence identity with human TCFL5. Gel-shift and yeast one-hybrid experiments showed that Tcfl5 interacts with a non-canonical CACGCG site that is present in the Clgn promoter. By using northern blot, RT-PCR and in situ hybridization, mouse Tcfl5 mRNA was detected only in testis, with the highest expression level in primary spermatocytes and round spermatids. The highest level of Tcfl5 protein was found in primary spermatocytes at the diplotene stage of meiotic prophase, where the protein colocalizes with transcriptionally active chromatin.
Figures






Similar articles
-
Identification of BOIP, a novel cDNA highly expressed during spermatogenesis that encodes a protein interacting with the orange domain of the hairy-related transcription factor HRT1/Hey1 in Xenopus and mouse.Dev Dyn. 2003 Dec;228(4):716-25. doi: 10.1002/dvdy.10406. Dev Dyn. 2003. PMID: 14648848
-
The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein.EMBO J. 1996 Aug 15;15(16):4317-29. EMBO J. 1996. PMID: 8861960 Free PMC article.
-
Expression of murine poly(A) binding protein II gene in testis.IUBMB Life. 2000 Jan;49(1):57-61. doi: 10.1080/713803589. IUBMB Life. 2000. PMID: 10772342
-
Testis-specific transcriptional control.Gene. 2004 Dec 8;343(1):11-22. doi: 10.1016/j.gene.2004.08.021. Gene. 2004. PMID: 15563828 Review.
-
Transcription factors 2: helix-loop-helix.Protein Profile. 1995;2(6):621-702. Protein Profile. 1995. PMID: 7553065 Review. No abstract available.
Cited by
-
Comparison of human B cell activation by TLR7 and TLR9 agonists.BMC Immunol. 2008 Jul 24;9:39. doi: 10.1186/1471-2172-9-39. BMC Immunol. 2008. PMID: 18652679 Free PMC article.
-
Comparative expression profiling of testis-enriched genes regulated during the development of spermatogonial cells.PLoS One. 2017 Apr 17;12(4):e0175787. doi: 10.1371/journal.pone.0175787. eCollection 2017. PLoS One. 2017. PMID: 28414809 Free PMC article.
-
Pseudoautosomal Region 1 Overdosage Affects the Global Transcriptome in iPSCs From Patients With Klinefelter Syndrome and High-Grade X Chromosome Aneuploidies.Front Cell Dev Biol. 2022 Feb 3;9:801597. doi: 10.3389/fcell.2021.801597. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35186953 Free PMC article.
-
Gestational arsenic exposure induces site-specific DNA hypomethylation in active retrotransposon subfamilies in offspring sperm in mice.Epigenetics Chromatin. 2020 Dec 2;13(1):53. doi: 10.1186/s13072-020-00375-3. Epigenetics Chromatin. 2020. PMID: 33267854 Free PMC article.
-
Tagged mutagenesis by efficient Minos-based germ line transposition.Mol Cell Biol. 2010 Jan;30(1):68-77. doi: 10.1128/MCB.00913-09. Mol Cell Biol. 2010. PMID: 19884347 Free PMC article.
References
-
- Baarends W.A. and Grootegoed,J.A. (1999) Molecular biology of male gametogenesis. In Fauser,B.C.J.M. (ed.), Molecular Biology in Reproductive Medicine. The Parthenon Publishing Group, NY, pp. 271–295.
-
- Eddy E.M. (2002) Male germ cell gene expression. Recent Prog. Horm. Res., 57, 103–128. - PubMed
-
- Wang P.J. (2004) X chromosomes, retrogenes and their role in male reproduction. Trends Endocrinol. Metab., 15, 79–83. - PubMed
-
- Oakberg E.F. (1956) Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat., 99, 507–516. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

