Orientation and mode of lipid-binding interaction of human apolipoprotein E C-terminal domain
- PMID: 15588256
- PMCID: PMC1135005
- DOI: 10.1042/BJ20041536
Orientation and mode of lipid-binding interaction of human apolipoprotein E C-terminal domain
Abstract
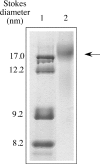
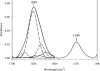
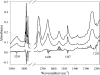
ApoE (apolipoprotein E) is an anti-atherogenic lipid transport protein that plays an integral role in lipoprotein metabolism and cholesterol homoeostasis. Lipid association educes critical functional features of apoE, mediating reduction in plasma and cellular cholesterol levels. The 10-kDa CT (C-terminal) domain of apoE facilitates helix-helix interactions in lipid-free state to promote apoE self-association and helix-lipid interactions during binding with lipoproteins, although the mode of lipid-binding interaction is not well understood. We investigated the mode of lipid-binding interaction and orientation of apoE CT domain on reconstituted lipoproteins. Isolated recombinant human apoE CT domain (residues 201-299) possesses a strong ability to interact with phospholipid vesicles, yielding lipoprotein particles with an apparent molecular mass of approximately 600 kDa, while retaining the overall alpha-helical content. Electron microscopy and non-denaturing PAGE analysis of DMPC (dimyristoylphosphatidylcholine)--apoE CT domain lipoprotein complexes revealed discoidal complexes with a diameter of approx. 17 nm. Cross-linking apoE CT domain on discoidal particles yielded dimeric species as the major product. Attenuated total reflectance Fourier transform IR spectroscopy of phospholipid-apoE CT domain complexes reveals that the helical axis is oriented perpendicular to fatty acyl chains of the phospholipid. Fluorescence quenching analysis of DMPC-apoE CT domain discoidal complexes by spin-labelled stearic acid indicated a relatively superficial location of the native tryptophan residues with respect to the plane of the phospholipid bilayer. Taken together, we propose that apoE CT domain interacts with phospholipid vesicles, forming a long extended helix that circumscribes the discoidal bilayer lipoprotein complex.
Figures




References
-
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. - PubMed
-
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
-
- Fielding C. J., Fielding P. E. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995;36:211–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

