Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage
- PMID: 15588287
- PMCID: PMC539292
- DOI: 10.1186/1742-2094-1-24
Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage
Abstract
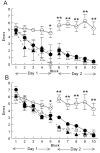
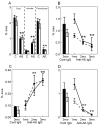
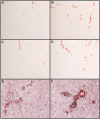
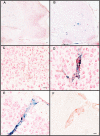
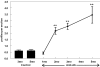
BACKGROUND: Anti-Abeta immunotherapy in transgenic mice reduces both diffuse and compact amyloid deposits, improves memory function and clears early-stage phospho-tau aggregates. As most Alzheimer disease cases occur well past midlife, the current study examined adoptive transfer of anti-Abeta antibodies to 19- and 23-month old APP-transgenic mice. METHODS: We investigated the effects of weekly anti-Abeta antibody treatment on radial-arm water-maze performance, parenchymal and vascular amyloid loads, and the presence of microhemorrhage in the brain. 19-month-old mice were treated for 1, 2 or 3 months while 23-month-old mice were treated for 5 months. Only the 23-month-old mice were subject to radial-arm water-maze testing. RESULTS: After 3 months of weekly injections, this passive immunization protocol completely reversed learning and memory deficits in these mice, a benefit that was undiminished after 5 months of treatment. Dramatic reductions of diffuse Abeta immunostaining and parenchymal Congophilic amyloid deposits were observed after five months, indicating that even well-established amyloid deposits are susceptible to immunotherapy. However, cerebral amyloid angiopathy increased substantially with immunotherapy, and some deposits were associated with microhemorrhage. Reanalysis of results collected from an earlier time-course study demonstrated that these increases in vascular deposits were dependent on the duration of immunotherapy. CONCLUSIONS: The cognitive benefits of passive immunotherapy persist in spite of the presence of vascular amyloid and small hemorrhages. These data suggest that clinical trials evaluating such treatments will require precautions to minimize potential adverse events associated with microhemorrhage.
Figures
References
-
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. - DOI - PubMed
-
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

