Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype
- PMID: 15590631
- PMCID: PMC2647591
- DOI: 10.1074/jbc.M412911200
Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype
Abstract
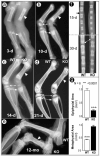
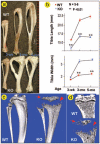
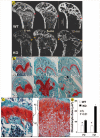
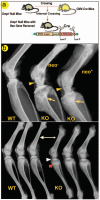
Understanding the molecular mechanisms by which cartilage formation is regulated is essential toward understanding the physiology of both embryonic bone development and postnatal bone growth. Although much is known about growth factor signaling in cartilage formation, the regulatory role of noncollagenous matrix proteins in this process are still largely unknown. In the present studies, we present evidence for a critical role of DMP1 (dentin matrix protein 1) in postnatal chondrogenesis. The Dmp1 gene was originally identified from a rat incisor cDNA library and has been shown to play an important role in late stage dentinogenesis. Whereas no apparent abnormalities were observed in prenatal bone development, Dmp1-deficient (Dmp1(-/-)) mice unexpectedly develop a severe defect in cartilage formation during postnatal chondrogenesis. Vertebrae and long bones in Dmp1-deficient (Dmp1(-/-)) mice are shorter and wider with delayed and malformed secondary ossification centers and an irregular and highly expanded growth plate, results of both a highly expanded proliferation and a highly expanded hypertrophic zone creating a phenotype resembling dwarfism with chondrodysplasia. This phenotype appears to be due to increased cell proliferation in the proliferating zone and reduced apoptosis in the hypertrophic zone. In addition, blood vessel invasion is impaired in the epiphyses of Dmp1(-/-) mice. These findings show that DMP1 is essential for normal postnatal chondrogenesis and subsequent osteogenesis.
Figures


References
-
- Kronenberg HM. Nature. 2003;423:332–336. - PubMed
-
- de Crombrugghe B, Lefebvre V, Nakashima K. Curr. Opin. Cell Biol. 2001;13:721–727. - PubMed
-
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Cell. 1997;89:747–754. - PubMed
-
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Cell. 1997;89:755–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

