Metagenomics: application of genomics to uncultured microorganisms
- PMID: 15590779
- PMCID: PMC539003
- DOI: 10.1128/MMBR.68.4.669-685.2004
Metagenomics: application of genomics to uncultured microorganisms
Abstract
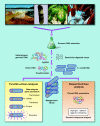
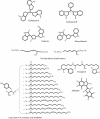
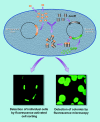
Metagenomics (also referred to as environmental and community genomics) is the genomic analysis of microorganisms by direct extraction and cloning of DNA from an assemblage of microorganisms. The development of metagenomics stemmed from the ineluctable evidence that as-yet-uncultured microorganisms represent the vast majority of organisms in most environments on earth. This evidence was derived from analyses of 16S rRNA gene sequences amplified directly from the environment, an approach that avoided the bias imposed by culturing and led to the discovery of vast new lineages of microbial life. Although the portrait of the microbial world was revolutionized by analysis of 16S rRNA genes, such studies yielded only a phylogenetic description of community membership, providing little insight into the genetics, physiology, and biochemistry of the members. Metagenomics provides a second tier of technical innovation that facilitates study of the physiology and ecology of environmental microorganisms. Novel genes and gene products discovered through metagenomics include the first bacteriorhodopsin of bacterial origin; novel small molecules with antimicrobial activity; and new members of families of known proteins, such as an Na(+)(Li(+))/H(+) antiporter, RecA, DNA polymerase, and antibiotic resistance determinants. Reassembly of multiple genomes has provided insight into energy and nutrient cycling within the community, genome structure, gene function, population genetics and microheterogeneity, and lateral gene transfer among members of an uncultured community. The application of metagenomic sequence information will facilitate the design of better culturing strategies to link genomic analysis with pure culture studies.
Figures


References
-
- Arp, A. J., M. L. Doyle, E. Di Cera, and S. J. Gill. 1990. Oxygenation properties of the two co-occurring hemoglobins of the tube worm Riftia pachyptila. Respir. Physiol. 80:323-334. - PubMed
-
- August, P. R., T. H. Grossman, C. Minor, M. P. Draper, I. A. MacNeil, J. M. Pemberton, K. M. Call, D. Holt, and M. S. Osburne. 2000. Sequence analysis and functional characterization of the violacein biosynthetic pathway from Chromobacterium violaceum. J. Mol. Microbiol. Biotechnol. 2:513-519. - PubMed
-
- Baker, B. J., and J. F. Banfield. 2003. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 44:139-152. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

