Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade
- PMID: 15590822
- PMCID: PMC539029
- DOI: 10.1128/EC.3.6.1476-1491.2004
Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade
Abstract
The evolutionarily conserved cyclic AMP (cAMP) signaling pathway controls cell functions in response to environmental cues in organisms as diverse as yeast and mammals. In the basidiomycetous human pathogenic fungus Cryptococcus neoformans, the cAMP pathway governs virulence and morphological differentiation. Here we identified and characterized adenylyl cyclase-associated protein, Aca1, which functions in parallel with the Galpha subunit Gpa1 to control the adenylyl cyclase (Cac1). Aca1 interacted with the C terminus of Cac1 in the yeast two-hybrid system. By molecular and genetic approaches, Aca1 was shown to play a critical role in mating by regulating cell fusion and filamentous growth in a cAMP-dependent manner. Aca1 also regulates melanin and capsule production via the Cac1-cAMP-protein kinase A pathway. Genetic epistasis studies support models in which Aca1 and Gpa1 are necessary and sufficient components that cooperate to activate adenylyl cyclase. Taken together, these studies further define the cAMP signaling cascade controlling virulence of this ubiquitous human fungal pathogen.
Figures
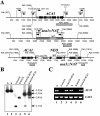




 , YSB166), and cac1Δ mutant (▵, YSB42) were glucose starved for 2 h. After the readdition of glucose, a portion of cell suspension was extracted, and its intracellular cAMP concentration was measured at the indicated times as described in Materials and Methods. Each datum point and error bar indicate the mean and standard deviation, respectively, for duplicate samples of two independent experiments. At all time points except zero, the cAMP levels were significantly higher in the wild-type or aca1Δ+ACA1 reconstituted strains than in the aca1Δ, gpa1Δ, aca1Δ gpa1Δ, or cac1Δ mutants (P < 0.05, as analyzed by using the Bonferroni multiple comparison test).
, YSB166), and cac1Δ mutant (▵, YSB42) were glucose starved for 2 h. After the readdition of glucose, a portion of cell suspension was extracted, and its intracellular cAMP concentration was measured at the indicated times as described in Materials and Methods. Each datum point and error bar indicate the mean and standard deviation, respectively, for duplicate samples of two independent experiments. At all time points except zero, the cAMP levels were significantly higher in the wild-type or aca1Δ+ACA1 reconstituted strains than in the aca1Δ, gpa1Δ, aca1Δ gpa1Δ, or cac1Δ mutants (P < 0.05, as analyzed by using the Bonferroni multiple comparison test).
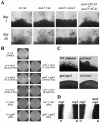
 ; YSB42, ten mice) strains by intranasal inhalation. The percent survival was monitored for 40 days postinfection. The median survival for animals infected with each strain was 23 days for the WT strain and the aca1Δ+ACA1 reconstituted strain and >40 days for the cac1Δ and the aca1Δ mutant strains.
; YSB42, ten mice) strains by intranasal inhalation. The percent survival was monitored for 40 days postinfection. The median survival for animals infected with each strain was 23 days for the WT strain and the aca1Δ+ACA1 reconstituted strain and >40 days for the cac1Δ and the aca1Δ mutant strains.
References
-
- Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. - PubMed
-
- Alspaugh, J. A., R. Pukkila-Worley, T. Harashima, L. M. Cavallo, D. Funnell, G. M. Cox, J. R. Perfect, J. W. Kronstad, and J. Heitman. 2002. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1:75-84. - PMC - PubMed
-
- Bockmühl, D. P., S. Krishnamurthy, M. Gerads, A. Sonneborn, and J. F. Ernst. 2001. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 42:1243-1257. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

