Cellular localization and role of kinase activity of PMK1 in Magnaporthe grisea
- PMID: 15590826
- PMCID: PMC539019
- DOI: 10.1128/EC.3.6.1525-1532.2004
Cellular localization and role of kinase activity of PMK1 in Magnaporthe grisea
Abstract
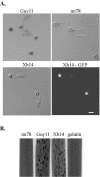
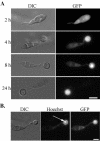
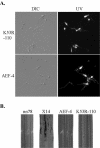
A mitogen-activated protein (MAP) kinase gene, PMK1, is known to regulate appressorium formation and infectious hyphal growth in the rice blast fungus Magnaporthe grisea. In this study, we constructed a green fluorescent protein gene-PMK1 fusion (GFP-PMK1) to examine the expression and localization of PMK1 in M. grisea during infection-related morphogenesis. The GFP-PMK1 fusion encoded a functional protein that complemented the defect of the pmk1 deletion mutant in appressorium formation and plant infection. Although a weak GFP signal was detectable in vegetative hyphae, conidia, and germ tubes, the expression of GFP-Pmk1 was increased in appressoria and developing conidia. Nuclear localization of GFP-Pmk1 proteins was observed in a certain percentage of appressoria. A kinase-inactive allele and a nonphosphorylatable allele of PMK1 were constructed by site-directed mutagenesis. Expression of these mutant PMK1 alleles did not complement the pmk1 deletion mutant. These data confirm that kinase activity and activation of PMK1 by the upstream MAP kinase kinase are required for appressorium formation and plant infection in M. grisea. When overexpressed with the RP27 promoter in the wild-type strain, both the kinase-inactive and nonphosphorylatable PMK1 fusion proteins caused abnormal germ tube branching. Overexpression of these PMK1 mutant alleles may interfere with the function of native PMK1 during appressorium formation.
Figures




References
-
- Blackwell, E., I. M. Halatek, H. J. N. Kim, A. T. Ellicott, A. A. Obukhov, and D. E. Stone. 2003. Effect of the pheromone-responsive G(alpha) and phosphatase proteins of Saccharomyces cerevisiae on the subcellular localization of the Fus3 mitogen-activated protein kinase. Mol. Cell. Biol. 23:1135-1150. - PMC - PubMed
-
- Bourett, T. M., J. A. Sweigard, K. J. Czymmek, A. Carroll, and R. J. Howard. 2002. Reef coral fluorescent proteins for visualizing fungal pathogens. Fungal Genet. Biol. 37:211-220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

