Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain
- PMID: 15590916
- PMCID: PMC6730274
- DOI: 10.1523/JNEUROSCI.3823-04.2004
Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain
Abstract
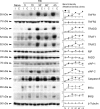
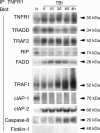
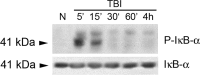
The tumor necrosis factor (TNF) ligand-receptor system plays an essential role in apoptosis that contributes to secondary damage after traumatic brain injury (TBI). TNF also stimulates inflammation by activation of gene transcription through the IkappaB kinase (IKK)/NF-kappaB and JNK (c-Jun N-terminal protein kinase)/AP-1 signaling cascades. The mechanism by which TNF signals between cell death and survival and the role of receptor localization in the activation of downstream signaling events are not fully understood. Here, TNF receptor 1 (TNFR1) signaling complexes in lipid rafts were investigated in the cerebral cortex of adult male Sprague Dawley rats subjected to moderate (1.8-2.2 atmospheres) fluid-percussion TBI and naive controls. In the normal rat cortex, a portion of TNFR1 was present in lipid raft microdomains, where it associated with the adaptor proteins TRADD (TNF receptor-associated death domain), TNF receptor-associated factor-2 (TRAF-2), the Ser/Thr kinase RIP (receptor-interacting protein), TRAF1, and cIAP-1 (cellular inhibitor of apoptosis protein-1), forming a survival signaling complex. Moderate TBI resulted in rapid recruitment of TNFR1, but not TNFR2 or Fas, to lipid rafts and induced alterations in the composition of signaling intermediates. TNFR1 and TRAF1 were polyubiquitinated in lipid rafts after TBI. Subsequently, the signaling complex contained activated caspase-8, thus initiating apoptosis. In addition, TBI caused a transient activation of NF-kappaB, but receptor signaling interacting proteins IKKalpha and IKKbeta were not detected in raft-containing fractions. Thus, redistribution of TNFR1 in lipid rafts and nonraft regions of the plasma membrane may regulate the diversity of signaling responses initiated by these receptors in the normal brain and after TBI.
Figures



Similar articles
-
Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage.Eur J Neurosci. 2006 Oct;24(8):2283-90. doi: 10.1111/j.1460-9568.2006.05123.x. Eur J Neurosci. 2006. PMID: 17074049
-
Spatial compartmentalization of tumor necrosis factor (TNF) receptor 1-dependent signaling pathways in human airway smooth muscle cells. Lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kappaB and MAPK pathways.J Biol Chem. 2006 Nov 10;281(45):34705-15. doi: 10.1074/jbc.M605738200. Epub 2006 Sep 18. J Biol Chem. 2006. PMID: 16982613 Free PMC article.
-
Protein Kinase-Mediated Decision Between the Life and Death.Adv Exp Med Biol. 2021;1275:1-33. doi: 10.1007/978-3-030-49844-3_1. Adv Exp Med Biol. 2021. PMID: 33539010
-
TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death.Biochem Pharmacol. 2016 Sep 15;116:1-10. doi: 10.1016/j.bcp.2016.03.009. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993379 Review.
-
Structural determinants of DISC function: new insights into death receptor-mediated apoptosis signalling.Pharmacol Ther. 2013 Nov;140(2):186-99. doi: 10.1016/j.pharmthera.2013.06.009. Epub 2013 Jul 8. Pharmacol Ther. 2013. PMID: 23845861 Review.
Cited by
-
Adipose tissue ATGL modifies the cardiac lipidome in pressure-overload-induced left ventricular failure.PLoS Genet. 2018 Jan 10;14(1):e1007171. doi: 10.1371/journal.pgen.1007171. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29320510 Free PMC article.
-
Bilateral gene interaction hierarchy analysis of the cell death gene response emphasizes the significance of cell cycle genes following unilateral traumatic brain injury.BMC Genomics. 2016 Feb 24;17:130. doi: 10.1186/s12864-016-2412-0. BMC Genomics. 2016. PMID: 26912237 Free PMC article.
-
Lipid rafts as signaling hubs in cancer cell survival/death and invasion: implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts.J Lipid Res. 2020 May;61(5):611-635. doi: 10.1194/jlr.TR119000439. Epub 2020 Nov 7. J Lipid Res. 2020. PMID: 33715811 Free PMC article. Review.
-
Decrease in tumor necrosis factor-alpha receptor-associated death domain results from ubiquitin-dependent degradation in obstructive renal injury in rats.Am J Pathol. 2009 Jul;175(1):74-83. doi: 10.2353/ajpath.2009.080884. Epub 2009 Jun 18. Am J Pathol. 2009. PMID: 19541932 Free PMC article.
-
Lipid rafts as a therapeutic target.J Lipid Res. 2020 May;61(5):687-695. doi: 10.1194/jlr.TR120000658. Epub 2020 Mar 23. J Lipid Res. 2020. PMID: 32205411 Free PMC article. Review.
References
-
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS (2003) A unified model for apical caspase activation. Mol Cell 11: 529-541. - PubMed
-
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP (1996) Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 2: 788-794. - PubMed
-
- Choi C, Benveniste EN (2004) Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Brain Res Rev 44: 65-81. - PubMed
-
- Clark WM, Lutsep HL (2001) Potential of anticytokine therapies in central nervous system ischaemia. Expert Opin Biol Ther 1: 227-237. - PubMed
-
- Cottin V, Doan JE, Riches DW (2002) Restricted localization of the TNF receptor CD120a to lipid rafts: a novel role for the death domain. J Immunol 168: 4095-4102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
