Sodium influx pathways during and after anoxia in rat hippocampal neurons
- PMID: 15590922
- PMCID: PMC6730285
- DOI: 10.1523/JNEUROSCI.2829-04.2004
Sodium influx pathways during and after anoxia in rat hippocampal neurons
Abstract
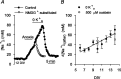
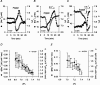
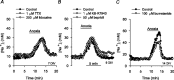
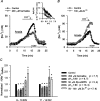
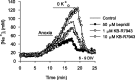
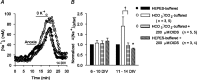
Mechanisms that contribute to Na+ influx during and immediately after 5 min anoxia were investigated in cultured rat hippocampal neurons loaded with the Na+-sensitive fluorophore sodium-binding benzofuran isophthalate. During anoxia, an influx of Na+ in the face of reduced Na+,K+-ATPase activity caused a rise in [Na+]i. After the return to normoxia, Na+,K+-ATPase activity mediated the recovery of [Na+]i despite continued Na+ entry. Sodium influx during and after anoxia occurred through multiple pathways and increased the longer neurons were maintained in culture. Under the experimental conditions used, Na+ entry during anoxia did not reflect the activation of ionotropic glutamate receptors, TTX- or lidocaine-sensitive Na+ channels, plasmalemmal Na+/Ca2+ exchange, Na+/H+ exchange, or HCO3--dependent mechanisms; rather, contributions were received from a Gd3+-sensitive pathway activated by reactive oxygen species and Na+/K+/2Cl- cotransport in neurons maintained for 6-10 and 11-14 d in vitro (DIV), respectively. Sodium entry immediately after anoxia was not attributable to the activation of ionotropic glutamate receptors, voltage-activated Na+ channels, or Na+/K+/2Cl- cotransport; rather, it occurred via Na+/Ca2+ exchange, Na+/H+ exchange, and a Gd3+-sensitive pathway similar to that observed during anoxia; 11-14 DIV neurons received an additional contribution from an -dependent mechanism(s). The results provide insight into the intrinsic mechanisms that contribute to disturbed internal Na+ homeostasis during and immediately after anoxia in rat hippocampal neurons and, in this way, may play a role in the pathogenesis of anoxic or ischemic cell injury.
Figures





Similar articles
-
Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons.Neuroscience. 1999;93(3):1003-16. doi: 10.1016/s0306-4522(99)00230-4. Neuroscience. 1999. PMID: 10473265
-
Regulation of intracellular sodium in cultured rat hippocampal neurones.J Physiol. 1997 Mar 15;499 ( Pt 3)(Pt 3):573-87. doi: 10.1113/jphysiol.1997.sp021951. J Physiol. 1997. PMID: 9130155 Free PMC article.
-
Early metabolic inhibition-induced intracellular sodium and calcium increase in rat cerebellar granule cells.J Physiol. 1999 Feb 15;515 ( Pt 1)(Pt 1):133-46. doi: 10.1111/j.1469-7793.1999.133ad.x. J Physiol. 1999. PMID: 9925884 Free PMC article.
-
Intracellular Na+ regulation in cardiac myocytes.Cardiovasc Res. 2003 Mar 15;57(4):897-912. doi: 10.1016/s0008-6363(02)00656-9. Cardiovasc Res. 2003. PMID: 12650868 Review.
-
Sodium ion transporters as new therapeutic targets in heart failure.Cardiovasc Hematol Agents Med Chem. 2008 Oct;6(4):229-36. doi: 10.2174/187152508785909546. Cardiovasc Hematol Agents Med Chem. 2008. PMID: 18855635 Review.
Cited by
-
Excessive Na+/H+ exchange in disruption of dendritic Na+ and Ca2+ homeostasis and mitochondrial dysfunction following in vitro ischemia.J Biol Chem. 2010 Nov 5;285(45):35155-68. doi: 10.1074/jbc.M110.101212. Epub 2010 Sep 3. J Biol Chem. 2010. PMID: 20817726 Free PMC article.
-
The importance of systemic response in the pathobiology of blast-induced neurotrauma.Front Neurol. 2010 Dec 10;1:151. doi: 10.3389/fneur.2010.00151. eCollection 2010. Front Neurol. 2010. PMID: 21206523 Free PMC article.
-
A dangerous liaison: Spreading depolarization and tissue acidification in cerebral ischemia.J Cereb Blood Flow Metab. 2025 Feb;45(2):201-218. doi: 10.1177/0271678X241289756. Epub 2024 Nov 13. J Cereb Blood Flow Metab. 2025. PMID: 39535276 Review.
-
THE ROLE OF INTRACELLULAR SODIUM (Na) IN THE REGULATION OF CALCIUM (Ca)-MEDIATED SIGNALING AND TOXICITY.Health (Irvine Calif). 2010;2(1):8-15. doi: 10.4236/health.2010.21002. Health (Irvine Calif). 2010. PMID: 21243124 Free PMC article.
-
Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it?Prog Neurobiol. 2010 Apr;90(4):439-70. doi: 10.1016/j.pneurobio.2009.12.007. Epub 2009 Dec 28. Prog Neurobiol. 2010. PMID: 20036308 Free PMC article. Review.
References
-
- Aarts M, Iihara K, Wei W-L, Xiong K-G, Arundine M, Cerwinski W, MacDonald JF, Tymianski M (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115: 863-877. - PubMed
-
- Anderson SE, Dickinson CZ, Liu H, Cala PM (1996) Effects of Na-K-2Cl cotransport inhibition on myocardial Na and Ca during ischemia and reperfusion. Am J Physiol 270: C608-C618. - PubMed
-
- Attaphitaya S, Park K, Melvin JE (1999) Molecular cloning and functional expression of a rat Na+/H+ exchanger (NHE5) highly expressed in brain. J Biol Chem 274: 4383-4388. - PubMed
-
- Avkiran M (2001) Protection of the ischemic myocardium by Na+/H+ exchange inhibitors: potential mechanisms of action. Basic Res Cardiol 96: 306-311. - PubMed
-
- Balestrino M, Lensman M, Parodi M, Perasso L, Rebaudo R, Melani R, Polenov S, Cupello A (2002) Role of creatine and phosphocreatine in neuronal protection from anoxic and ischemic damage. Amino Acids 23: 221-229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
