Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe
- PMID: 15590927
- PMCID: PMC6730268
- DOI: 10.1523/JNEUROSCI.3677-04.2004
Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe
Abstract
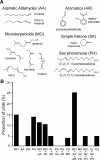
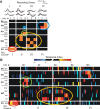
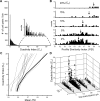
In the insect antennal lobe, odor discrimination depends on the ability of the brain to read neural activity patterns across arrays of uniquely identifiable olfactory glomeruli. Less is understood about the complex temporal dynamics and interglomerular interactions that underlie these spatial patterns. Using neural-ensemble recording, we show that the evoked firing patterns within and between groups of glomeruli are odor dependent and organized in both space and time. Simultaneous recordings from up to 15 units per ensemble were obtained from four zones of glomerular neuropil in response to four classes of odorants: pheromones, monoterpenoids, aromatics, and aliphatics. Each odor class evoked a different pattern of excitation and inhibition across recording zones. The excitatory response field for each class was spatially defined, but inhibitory activity was spread across the antennal lobe, reflecting a center-surround organization. Some chemically related odorants were not easily distinguished by their spatial patterns, but each odorant evoked transient synchronous firing across a uniquely different subset of ensemble units. Examination of 535 cell pairs revealed a strong relationship between their recording positions, temporal correlations, and similarity of odor response profiles. These findings provide the first definitive support for a nested architecture in the insect olfactory system that uses both spatial and temporal coordination of firing to encode chemosensory signals. The spatial extent of the representation is defined by a stereotyped focus of glomerular activity for each odorant class, whereas the transient temporal correlations embedded within the ensemble provide a second coding dimension that can facilitate discrimination between chemically similar volatiles.
Figures





Similar articles
-
Multi-unit recordings reveal context-dependent modulation of synchrony in odor-specific neural ensembles.Nat Neurosci. 2000 Sep;3(9):927-31. doi: 10.1038/78840. Nat Neurosci. 2000. PMID: 10966624
-
Combinatorial odor discrimination in the brain: attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli.J Comp Neurol. 1998 Oct 12;400(1):35-56. J Comp Neurol. 1998. PMID: 9762865
-
Temporal tuning of odor responses in pheromone-responsive projection neurons in the brain of the sphinx moth Manduca sexta.J Comp Neurol. 1999 Jun 21;409(1):1-12. J Comp Neurol. 1999. PMID: 10363707
-
Representation of primary plant odorants in the antennal lobe of the moth Heliothis virescens using calcium imaging.Chem Senses. 2004 Mar;29(3):253-67. doi: 10.1093/chemse/bjh026. Chem Senses. 2004. PMID: 15047600 Review.
-
Olfactory encoding within the insect antennal lobe: The emergence and role of higher order temporal correlations in the dynamics of antennal lobe spiking activity.J Theor Biol. 2021 Aug 7;522:110700. doi: 10.1016/j.jtbi.2021.110700. Epub 2021 Apr 2. J Theor Biol. 2021. PMID: 33819477 Review.
Cited by
-
A 4-dimensional representation of antennal lobe output based on an ensemble of characterized projection neurons.J Neurosci Methods. 2009 Jun 15;180(2):208-23. doi: 10.1016/j.jneumeth.2009.03.019. Epub 2009 Mar 31. J Neurosci Methods. 2009. PMID: 19464513 Free PMC article.
-
Encoding of antennal position and velocity by the Johnston's organ in hawkmoths.J Exp Biol. 2025 May 1;228(9):jeb249342. doi: 10.1242/jeb.249342. Epub 2025 May 2. J Exp Biol. 2025. PMID: 40099381 Free PMC article.
-
Effects of Mechanosensory Input on the Tracking of Pulsatile Odor Stimuli by Moth Antennal Lobe Neurons.Front Neurosci. 2021 Oct 7;15:739730. doi: 10.3389/fnins.2021.739730. eCollection 2021. Front Neurosci. 2021. PMID: 34690678 Free PMC article.
-
Is there a space-time continuum in olfaction?Cell Mol Life Sci. 2009 Jul;66(13):2135-50. doi: 10.1007/s00018-009-0011-9. Epub 2009 Mar 18. Cell Mol Life Sci. 2009. PMID: 19294334 Free PMC article. Review.
-
Similar odorants elicit different behavioral and physiological responses, some supersustained.J Neurosci. 2011 May 25;31(21):7891-9. doi: 10.1523/JNEUROSCI.6254-10.2011. J Neurosci. 2011. PMID: 21613503 Free PMC article.
References
-
- Aertsen AMHJ, Gerstein GL, Habib M, Palm G (1989) Dynamics of neuronal firing correlation: modulation of “effective connectivity.” J Neurophysiol 61: 900-917. - PubMed
-
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT (2003) Centre-surround inhibition among olfactory bulb glomeruli. Nature 426: 623-629. - PubMed
-
- Brown EN, Kass RE, Mitra PP (2004) Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci 7: 456-461. - PubMed
-
- Buonviso N, Chaput MA (1990) Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: electrophysiological study using simultaneous single unit recordings. J Neurophysiol 63: 447-454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
