Global forebrain dynamics predict rat behavioral states and their transitions
- PMID: 15590930
- PMCID: PMC6730270
- DOI: 10.1523/JNEUROSCI.3524-04.2004
Global forebrain dynamics predict rat behavioral states and their transitions
Abstract
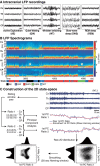
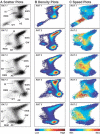
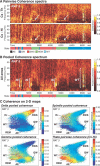
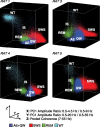
The wake-sleep cycle, a spontaneous succession of global brain states that correspond to major overt behaviors, occurs in all higher vertebrates. The transitions between these states, at once rapid and drastic, remain poorly understood. Here, intracranial local field potentials (LFPs) recorded in the cortex, hippocampus, striatum, and thalamus were used to characterize the neurophysiological correlates of the rat wake-sleep cycle. By way of a new method for the objective classification and quantitative investigation of all major brain states, we demonstrate that global brain state transitions occur simultaneously across multiple forebrain areas as specific spectral trajectories with characteristic path, duration, and coherence bandwidth. During state transitions, striking changes in neural synchronization are effected by the prominent narrow-band LFP oscillations that mark state boundaries. Our results demonstrate that distant forebrain areas tightly coordinate the processing of neural information during and between global brain states, indicating a very high degree of functional integration across the entire wake-sleep cycle. We propose that transient oscillatory synchronization of synaptic inputs, which underlie the rapid switching of global brain states, may facilitate the exchange of information within and across brain areas at the boundaries of very distinct neural processing regimens.
Figures



Similar articles
-
Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action.Neuroscience. 2001;106(4):699-715. doi: 10.1016/s0306-4522(01)00319-0. Neuroscience. 2001. PMID: 11682157
-
Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system.J Sleep Res. 2004 Sep;13(3):179-208. doi: 10.1111/j.1365-2869.2004.00412.x. J Sleep Res. 2004. PMID: 15339255 Review.
-
Sleep-waking states develop independently in the isolated forebrain and brain stem following early postnatal midbrain transection in cats.Neuroscience. 2001;106(4):717-31. doi: 10.1016/s0306-4522(01)00329-3. Neuroscience. 2001. PMID: 11682158
-
Gamma band directional interactions between basal forebrain and visual cortex during wake and sleep states.J Physiol Paris. 2016 Sep;110(1-2):19-28. doi: 10.1016/j.jphysparis.2016.11.011. Epub 2016 Nov 29. J Physiol Paris. 2016. PMID: 27913167
-
[Selective stimulations and lesions of the rat brain nuclei as the models for research of the human sleep pathology mechanisms].Glas Srp Akad Nauka Med. 2011;(51):85-97. Glas Srp Akad Nauka Med. 2011. PMID: 22165729 Review. Serbian.
Cited by
-
Phenomenology and connectionism.Front Psychol. 2011 Nov 15;2:288. doi: 10.3389/fpsyg.2011.00288. eCollection 2011. Front Psychol. 2011. PMID: 22121354 Free PMC article.
-
Apical amplification-a cellular mechanism of conscious perception?Neurosci Conscious. 2021 Oct 13;2021(2):niab036. doi: 10.1093/nc/niab036. eCollection 2021. Neurosci Conscious. 2021. PMID: 34650815 Free PMC article.
-
Different subtypes of striatal neurons are selectively modulated by cortical oscillations.J Neurosci. 2009 Apr 8;29(14):4571-85. doi: 10.1523/JNEUROSCI.5097-08.2009. J Neurosci. 2009. PMID: 19357282 Free PMC article.
-
Dynamics of recovery from anaesthesia-induced unconsciousness across primate neocortex.Brain. 2020 Mar 1;143(3):833-843. doi: 10.1093/brain/awaa017. Brain. 2020. PMID: 32049333 Free PMC article.
-
Arousal increases the representational capacity of cortical tissue.J Comput Neurosci. 2009 Oct;27(2):211-27. doi: 10.1007/s10827-009-0138-6. Epub 2009 Mar 27. J Comput Neurosci. 2009. PMID: 19326198
References
-
- Achermann P, Borbely AA (1997) Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81: 213-222. - PubMed
-
- Achermann P, Borbely AA (1998a) Coherence analysis of the human sleep electroencephalogram. Neuroscience 85: 1195-1208. - PubMed
-
- Achermann P, Borbely AA (1998b) Temporal evolution of coherence and power in the human sleep electroencephalogram. J Sleep Res 7 [Suppl 1]: 36-41. - PubMed
-
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA (1997) An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods 73: 69-79. - PubMed
-
- Amzica F, Steriade M (1995) Short- and long-range neuronal synchronization of the slow (<1 Hz) cortical oscillation. J Neurophysiol 73: 20-38. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
