Role of alpha-synuclein in presynaptic dopamine recruitment
- PMID: 15590933
- PMCID: PMC6730279
- DOI: 10.1523/JNEUROSCI.2559-04.2004
Role of alpha-synuclein in presynaptic dopamine recruitment
Abstract
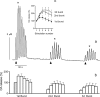
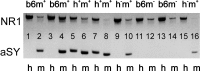
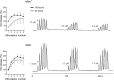
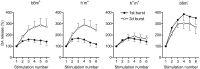
Real-time monitoring of stimulated dopamine release in mice with different alpha-synuclein expression was used to study the role of alpha-synuclein in presynaptic dopamine recruitment. Repeated electrical stimulations of ascending dopaminergic pathways decreased the capacity of the readily releasable pool (RRP) and temporarily increased its refilling rate, significantly slowing the rate of dopamine decline in mice with normally expressed alpha-synuclein. Mice with alpha-synuclein null mutation demonstrated a permanent increase of the refilling rate. This increase maintained stable dopamine release during stimulation (which induced dopamine decline in other animals) and served as an adaptation to altered dopamine compartmentalization. Mice without alpha-synuclein and with overexpression of human A30P mutated alpha-synuclein had a lower capacity of the dopamine storage pool than other animals. Reducing capacity of the storage pool in transgenic A30P mice led to paradoxical effects of l-dopa, which elevated dopamine release in response to single stimulation but decreased the refilling rate of the RRP.
Figures

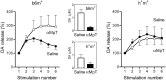
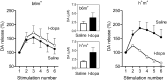
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239-252. - PubMed
-
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci 22: 8797-8807. - PMC - PubMed
-
- Ewing AG, Bigelow JC, Wightman RM (1983) Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science 221: 169-171. - PubMed
-
- Jakala P, Puolivali J, Kerokoski P, van Groen T, Beyreuther K, Hartmann T (2002) Intracellular a-synuclein (A-SYN) accumulation in transgenic mice expressing A30P a-synuclein mutation. Neurobiol Aging 23: S252.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
