Maurocalcine and domain A of the II-III loop of the dihydropyridine receptor Cav 1.1 subunit share common binding sites on the skeletal ryanodine receptor
- PMID: 15591063
- PMCID: PMC2712624
- DOI: 10.1074/jbc.C400433200
Maurocalcine and domain A of the II-III loop of the dihydropyridine receptor Cav 1.1 subunit share common binding sites on the skeletal ryanodine receptor
Abstract
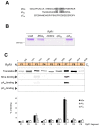
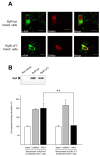
Maurocalcine is a scorpion venom toxin of 33 residues that bears a striking resemblance to the domain A of the dihydropyridine voltage-dependent calcium channel type 1.1 (Cav1.1) subunit. This domain belongs to the II-III loop of Cav1.1, which is implicated in excitation-contraction coupling. Besides the structural homology, maurocalcine also modulates RyR1 channel activity in a manner akin to a synthetic peptide of domain A. Because of these similarities, we hypothesized that maurocalcine and domain A may bind onto an identical region(s) of RyR1. Using a set of RyR1 fragments, we demonstrate that peptide A and maurocalcine bind onto two discrete RyR1 regions: fragments 3 and 7 encompassing residues 1021-1631 and 3201-3661, respectively. The binding onto fragment 7 is of greater importance and was thus further investigated. We found that the amino acid region 3351-3507 of RyR1 (fragment 7.2) is sufficient for these interactions. Proof that peptide A and maurocalcine bind onto the same site is provided by competition experiments in which binding of fragment 7.2 to peptide A is inhibited by preincubation with maurocalcine. Moreover, when expressed in COS-7 cells, RyR1 carrying a deletion of fragment 7 shows a loss of interaction with both peptide A and maurocalcine. At the functional level, this deletion abolishes the maurocalcine induced stimulation of [3H]ryanodine binding onto microsomes of transfected COS-7 cells without affecting the caffeine and ATP responses.
Figures

References
-
- Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Nature. 1996;380:72–75. - PubMed
-
- Chavis P, Fagni L, Lansman JB, Bockaert J. Nature. 1996;382:719–722. - PubMed
-
- Catterall WA. Science. 1991;253:1499–1500. - PubMed
-
- Tanabe T, Beam KG, Adams BA, Nicodome T, Numa S. Nature. 1990;346:567–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

