Phosphorylated BRCA1 is predominantly located in the nucleus and mitochondria
- PMID: 15591126
- PMCID: PMC545929
- DOI: 10.1091/mbc.e04-10-0895
Phosphorylated BRCA1 is predominantly located in the nucleus and mitochondria
Abstract
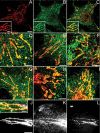
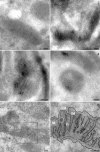
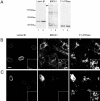
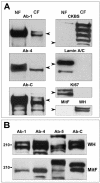
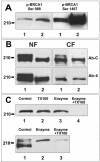
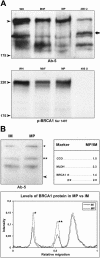
Multiple copies of the mitochondrial genome in eukaryotic cells are organized into protein-DNA complexes called nucleoids. Mitochondrial genome repair mechanisms have been reported, but they are less well characterized than their nuclear counterparts. To expand our knowledge of mitochondrial genome maintenance, we have studied the localization of the BRCA1 protein, known to be involved in nuclear repair pathways. Our confocal and immunoelectron microscopy results show that BRCA1 is present in mitochondria of several human cancer cell lines and in primary breast and nasal epithelial cells. BRCA1 localization in mitochondria frequently overlapped that of nucleoids. Small interfering RNA-mediated knockdown of BRCA1 in human cancer cells (confirmed by Western blot) results in decreased nuclear, cytoplasmic, and mitochondrial staining after immunofluorescence microscopy, establishing the specificity of the BRCA1 immunolabeling. Furthermore, using cell fractionation, dephosphorylation, and enzyme protection experiments, we show that a 220-kDa phosphorylated isoform of BRCA1 is enriched in mitochondrial and nuclear fractions but reduced in cytoplasmic subcellular fractions. Submitochondrial fractionation confirmed the presence of BRCA1 protein in isolated mitoplasts. Because phosphorylation of BRCA1 and subsequent changes in subcellular localization are known to follow DNA damage, our data support a universal role for BRCA1 in the maintenance of genome integrity in both mitochondria and nucleus.
Figures


Similar articles
-
DNA damage induces p53-dependent BRCA1 nuclear export.J Biol Chem. 2004 Jul 2;279(27):28574-84. doi: 10.1074/jbc.M404137200. Epub 2004 Apr 15. J Biol Chem. 2004. PMID: 15087457
-
BRCA1 protein and nucleolin colocalize in breast carcinoma tissue and cancer cell lines.Am J Pathol. 2010 Mar;176(3):1203-14. doi: 10.2353/ajpath.2010.081063. Epub 2010 Jan 14. Am J Pathol. 2010. PMID: 20075200 Free PMC article.
-
BARD1 translocation to mitochondria correlates with Bax oligomerization, loss of mitochondrial membrane potential, and apoptosis.J Biol Chem. 2007 Jul 13;282(28):20513-22. doi: 10.1074/jbc.M702627200. Epub 2007 May 17. J Biol Chem. 2007. PMID: 17510055
-
Regulation of BRCA1, BRCA2 and BARD1 intracellular trafficking.Bioessays. 2005 Sep;27(9):884-93. doi: 10.1002/bies.20277. Bioessays. 2005. PMID: 16108063 Review.
-
A nuclear function for the tumor suppressor BRCA1.Histol Histopathol. 2000 Jan;15(1):299-307. doi: 10.14670/HH-15.299. Histol Histopathol. 2000. PMID: 10668218 Review.
Cited by
-
BRCA1 is a novel regulator of metabolic function in skeletal muscle.J Lipid Res. 2014 Apr;55(4):668-80. doi: 10.1194/jlr.M043851. Epub 2014 Feb 24. J Lipid Res. 2014. PMID: 24565757 Free PMC article.
-
Mitochondria and familial predisposition to breast cancer.Curr Genomics. 2013 May;14(3):195-203. doi: 10.2174/1389202911314030005. Curr Genomics. 2013. PMID: 24179442 Free PMC article.
-
Environmental exposure and mitochondrial epigenetics: study design and analytical challenges.Hum Genet. 2014 Mar;133(3):247-57. doi: 10.1007/s00439-013-1417-x. Epub 2014 Jan 9. Hum Genet. 2014. PMID: 24402053 Free PMC article. Review.
-
Emerging functions of the Fanconi anemia pathway at a glance.J Cell Sci. 2017 Aug 15;130(16):2657-2662. doi: 10.1242/jcs.204909. J Cell Sci. 2017. PMID: 28811338 Free PMC article. Review.
-
Localization of BRCA1 protein in breast cancer tissue and cell lines with mutations.Cancer Cell Int. 2013 Jul 15;13(1):70. doi: 10.1186/1475-2867-13-70. Cancer Cell Int. 2013. PMID: 23855721 Free PMC article.
References
-
- Anderson, S., et al. (1981). Sequence and organization of the human mitochondrial genome. Nature 290, 457-465. - PubMed
-
- Bianchi, N. O., Bianchi, M. S., and Richard, S. M. (2001). Mitochondrial genome instability in human cancers. Mutat. Res. 488, 9-23. - PubMed
-
- Bourgeois, C. A., Hemon, D., and Bouteille, M. (1979). Structural relationship between the nucleolus and the nuclear envelope. J. Ultrastruct. Res. 68, 328-340. - PubMed
-
- Chen, C. F., Li, S., Chen, Y., Chen, P. L., Sharp, Z. D., and Lee, W. H. (1996). The nuclear localization sequences of the BRCA1 protein interact with the importin-alpha subunit of the nuclear transport signal receptor. J. Biol. Chem. 271, 32863-32868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

