Agonist-induced endocytosis of CC chemokine receptor 5 is clathrin dependent
- PMID: 15591129
- PMCID: PMC545921
- DOI: 10.1091/mbc.e04-08-0687
Agonist-induced endocytosis of CC chemokine receptor 5 is clathrin dependent
Abstract
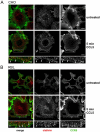
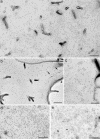
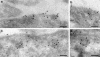
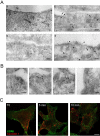
The signaling activity of several chemokine receptors, including CC chemokine receptor 5 (CCR5), is in part controlled by their internalization, recycling, and/or degradation. For CCR5, agonists such as the chemokine CCL5 induce internalization into early endosomes containing the transferrin receptor, a marker for clathrin-dependent endocytosis, but it has been suggested that CCR5 may also follow clathrin-independent routes of internalization. Here, we present a detailed analysis of the role of clathrin in chemokine-induced CCR5 internalization. Using CCR5-transfected cell lines, immunofluorescence, and electron microscopy, we demonstrate that CCL5 causes the rapid redistribution of scattered cell surface CCR5 into large clusters that are associated with flat clathrin lattices. Invaginated clathrin-coated pits could be seen at the edge of these lattices and, in CCL5-treated cells, these pits contain CCR5. Receptors internalized via clathrin-coated vesicles follow the clathrin-mediated endocytic pathway, and depletion of clathrin with small interfering RNAs inhibits CCL5-induced CCR5 internalization. We found no evidence for CCR5 association with caveolae during agonist-induced internalization. However, sequestration of cholesterol with filipin interferes with agonist binding to CCR5, suggesting that cholesterol and/or lipid raft domains play some role in the events required for CCR5 activation before internalization.
Figures

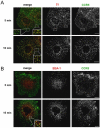

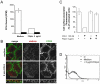

References
-
- Alkhatib, G., Locati, M., Kennedy, P. E., Murphy, P. M., and Berger, E. A. (1997). HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor down-modulation. Virology 234, 340-348. - PubMed
-
- Amara, A., LeGall, S., Schwartz, O., Salamero, J., Montes, M., Loetscher, P., Baggiolini, M., Virelizier, J. L., and Arenzana-Seisdedos, F. (1997). HIV coreceptor downregulation as antiviral principle: SDF-1 alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186, 139-146. - PMC - PubMed
-
- Aramori, I., Zhang, J., Ferguson, S. S., Bieniasz, P. D., Cullen, B. R., and Caron, M.G. (1997). Molecular mechanism of desensitization of the chemokine receptor CCR-5, receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO J. 16, 4606-4616. - PMC - PubMed
-
- Blanpain, C., et al. (2001). Palmitoylation of CCR5 is critical for receptor trafficking and efficient activation of intracellular signaling pathways. J. Biol. Chem. 276, 23795-23804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

