Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue
- PMID: 15591341
- PMCID: PMC539718
- DOI: 10.1073/pnas.0406230101
Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue
Abstract
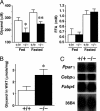
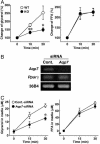
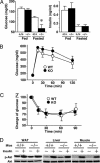
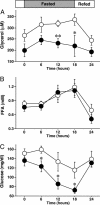
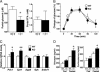
Adipocytes hydrolyze triglycerides and secrete free fatty acids and glycerol into the circulation. The molecular mechanism involved in glycerol transport from adipocytes has not been elucidated. Here, we investigated glycerol and glucose metabolism in mice lacking aquaporin 7 (Aqp7), a member of the aquaglyceroporins expressed in adipose tissue, and demonstrated that Aqp7 functions as a glycerol gateway molecule in vivo. Aqp7-knockout (KO) mice had lower plasma glycerol levels compared with WT mice but had normal plasma free fatty acid levels. The increase in plasma glycerol level in response to beta(3)-adrenergic agonist was severely impaired in KO mice. Epinephrine-stimulated glycerol secretion was also impaired in Aqp7 knockdown adipocytes. During prolonged fasting, plasma glycerol was elevated and the plasma glucose level was maintained in WT mice. In contrast, KO mice showed a disrupted increase of plasma glycerol and rapid reduction of plasma glucose during prolonged fasting. Our findings indicate that the lack of effective glycerol transport from adipocytes by glycerol gateway molecule causes defective adaptation to prolonged fasting.
Figures

References
-
- Denker, B. M., Smith, B. L., Kuhajda, F. P. & Agre, P. (1988) J. Biol. Chem. 263, 15634-15642. - PubMed
-
- Agre, P. & Kozono, D. (2003) FEBS Lett. 555, 72-78. - PubMed
-
- Preston, G. M., Carroll, T. P., Guggino, W. B. & Agre, P. (1992) Science 256, 385-387. - PubMed
-
- Giles, J. (2003) Nature 425, 651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

