A vesicle bioreactor as a step toward an artificial cell assembly
- PMID: 15591347
- PMCID: PMC539773
- DOI: 10.1073/pnas.0408236101
A vesicle bioreactor as a step toward an artificial cell assembly
Abstract
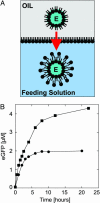
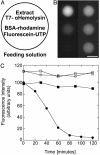
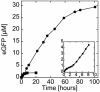
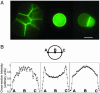
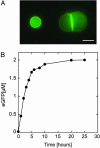
An Escherichia coli cell-free expression system is encapsulated in a phospholipid vesicle to build a cell-like bioreactor. Large unilamellar vesicles containing extracts are produced in an oil-extract emulsion. To form a bilayer the vesicles are transferred into a feeding solution that contains ribonucleotides and amino acids. Transcription-translation of plasmid genes is isolated in the vesicles. Whereas in bulk solution expression of enhanced GFP stops after 2 h, inside the vesicle permeability of the membrane to the feeding solution prolongs the expression for up to 5 h. To solve the energy and material limitations and increase the capacity of the reactor, the alpha-hemolysin pore protein from Staphylococcus aureus is expressed inside the vesicle to create a selective permeability for nutrients. The reactor can then sustain expression for up to 4 days with a protein production of 30 muM after 4 days. Oxygen diffusion and osmotic pressure are critical parameters to maintain expression and avoid vesicle burst.
Figures


References
-
- von Neumann, J. (1966) Theory of Self-Reproducing Automata (Univ. of Illinois Press, Champaign).
-
- Turing, A. M. (1936) Proc. London Math. Soc. Ser. 2 42, 230–265.
-
- Ganti, T. (2003) The Principles of Life (Oxford Univ. Press, New York).
-
- Pohorille, A. & Deamer, D. (2002) Trends Biotechnol. 20, 123–128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

