A quantitative assessment of models for voltage-dependent gating of ion channels
- PMID: 15591352
- PMCID: PMC539724
- DOI: 10.1073/pnas.0408116101
A quantitative assessment of models for voltage-dependent gating of ion channels
Erratum in
- Proc Natl Acad Sci U S A. 2005 Jul 26;102(30):10753
Abstract
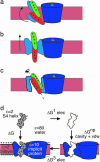
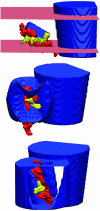
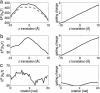
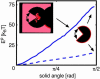
Voltage-gated ion channels open and close, or "gate," in response to changes in membrane potential. The electric field across the membrane-protein complex exerts forces on charged residues driving the channel into different functional conformations as the membrane potential changes. To act with the greatest sensitivity, charged residues must be positioned at key locations within or near the transmembrane region, which requires desolvating charged groups, a process that can be energetically prohibitive. Although there is good agreement on which residues are involved in this process for voltage-activated potassium channels, several different models of the sensor geometry and gating motions have been proposed. Here we incorporate low-resolution structural information about the channel into a Poisson-Boltzmann calculation to determine solvation barrier energies and gating charge values associated with each model. The principal voltage-sensing helix, S4, is represented explicitly, whereas all other regions are represented as featureless, dielectric media with complex boundaries. From our calculations, we conclude that a pure rotation of the S4 segment within the voltage sensor is incapable of producing the observed gating charge values, although this shortcoming can be partially remedied by first tipping and then minimally translating the S4 helix. Models in which the S4 segment has substantial interaction with the low-dielectric environment of the membrane incur solvation energies of hundreds of k(B)T, and activation times based on these energies are orders of magnitude slower than experimentally observed.
Figures

References
-
- Seoh, S. A., Sigg, D., Papazian, D. M. & Bezanilla, F. (1996) Neuron 16, 1159–1167. - PubMed
-
- Aggarwal, S. K. & MacKinnon, R. (1996) Neuron 16, 1169–1177. - PubMed
-
- Catterall, W. A. (1986) Annu. Rev. Biochem. 55, 953–985. - PubMed
-
- Cha, A., Snyder, G. E., Selvin, P. R. & Bezanilla, F. (1999) Nature 402, 809–813. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

