Comparative genomic hybridization using oligonucleotide microarrays and total genomic DNA
- PMID: 15591353
- PMCID: PMC535426
- DOI: 10.1073/pnas.0407979101
Comparative genomic hybridization using oligonucleotide microarrays and total genomic DNA
Abstract
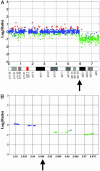
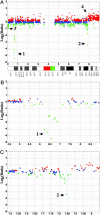
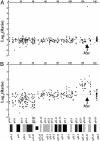
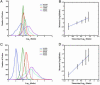
Array-based comparative genomic hybridization (CGH) measures copy-number variations at multiple loci simultaneously, providing an important tool for studying cancer and developmental disorders and for developing diagnostic and therapeutic targets. Arrays for CGH based on PCR products representing assemblies of BAC or cDNA clones typically require maintenance, propagation, replication, and verification of large clone sets. Furthermore, it is difficult to control the specificity of the hybridization to the complex sequences that are present in each feature of such arrays. To develop a more robust and flexible platform, we created probe-design methods and assay protocols that make oligonucleotide microarrays synthesized in situ by inkjet technology compatible with array-based comparative genomic hybridization applications employing samples of total genomic DNA. Hybridization of a series of cell lines with variable numbers of X chromosomes to arrays designed for CGH measurements gave median ratios for X-chromosome probes within 6% of the theoretical values (0.5 for XY/XX, 1.0 for XX/XX, 1.4 for XXX/XX, 2.1 for XXXX/XX, and 2.6 for XXXXX/XX). Furthermore, these arrays detected and mapped regions of single-copy losses, homozygous deletions, and amplicons of various sizes in different model systems, including diploid cells with a chromosomal breakpoint that has been mapped and sequenced to a precise nucleotide and tumor cell lines with highly variable regions of gains and losses. Our results demonstrate that oligonucleotide arrays designed for CGH provide a robust and precise platform for detecting chromosomal alterations throughout a genome with high sensitivity even when using full-complexity genomic samples.
Figures


References
-
- Pinkel, D., Segraves, R., Sudar, D., Clark, S., Poole, I., Kowbel, D., Collins, C., Kuo, W. L., Chen, C., Zhai, Y., et al. (1998) Nat. Genet. 20, 207-211. - PubMed
-
- Pollack, J. R., Perou, C. M., Alizadeh, A. A., Eisen, M. B., Pergamenschikov, A., Williams, C. F., Jeffrey, S. S., Botstein, D. & Brown, P. O. (1999) Nat. Genet. 23, 41-46. - PubMed
-
- Snijders, A. M., Nowak, N., Segraves, R., Blackwood, S., Brown, N., Conroy, J., Hamilton, G., Hindle, A. K., Huey, B., Kimura, K., et al. (2001) Nat. Genet. 29, 263-264. - PubMed
-
- Hodgson, G., Hager, J. H., Volik, S., Hariono, S., Wernick, M., Moore, D., Nowak, N., Albertson, D. G., Pinkel, D., Collins, C., et al. (2001) Nat. Genet. 29, 459-464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

