Human peripheral and gastric lymphocyte responses to Helicobacter pylori NapA and AphC differ in infected and uninfected individuals
- PMID: 15591500
- PMCID: PMC1774350
- DOI: 10.1136/gut.2003.025494
Human peripheral and gastric lymphocyte responses to Helicobacter pylori NapA and AphC differ in infected and uninfected individuals
Erratum in
- Gut. 2005 Apr;54(4):568. Morales, VA [corrected to Athie-Morales, V]
Abstract
Background: In this study, we identify the nature of the immunological response of human peripheral blood mononuclear cells (PBMC) and lamina propria gastric lymphocytes (LPL) to two Helicobacter pylori antigens, the neutrophil activating protein (NapA) and alkyl hydroperoxide reductase (AphC). These antigens were identified and selected for study based on the observation that serological recognition of these proteins was associated with H pylori negative status in humans.
Aims: The aim was to study the serological, proliferative, and cytokine responses of PBMC and LPL, obtained from H pylori infected and uninfected individuals, to these antigens.
Methods: Patient serum, PBMC, and LPL were used to determine antibody isotype, and proliferative and cytokine responses to recombinant forms of NapA and AphC using western blotting and ELISA.
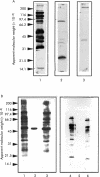
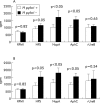
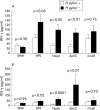
Results: Western blotting revealed antibody reactivity to recombinant NapA and AphC among the H pylori negative population studied. Both the proliferative and interferon gamma responses of PBMC and LPL to NapA and AphC were significantly higher in H pylori negative compared with H pylori positive subjects. Analysis of the IgG subclass profiles to both antigens revealed a T helper 1 associated IgG3 antibody response in uninfected individuals. However, interleukin 10 production was greater in H pylori positive individuals in response to these antigens.
Conclusions: Taken together these data are consistent with an immune response to these antigens skewed towards a T helper 1 response in the uninfected cohort.
Figures





Similar articles
-
gammadelta T cells increase with gastric mucosal interleukin (IL)-7, IL-1beta, and Helicobacter pylori urease specific immunoglobulin levels via CCR2 upregulation in Helicobacter pylori gastritis.J Gastroenterol Hepatol. 2006 Jan;21(1 Pt 1):32-40. doi: 10.1111/j.1440-1746.2005.04148.x. J Gastroenterol Hepatol. 2006. PMID: 16706809
-
Interleukin-12 drives the Th1 signaling pathway in Helicobacter pylori-infected human gastric mucosa.Infect Immun. 2007 Apr;75(4):1738-44. doi: 10.1128/IAI.01446-06. Epub 2007 Jan 12. Infect Immun. 2007. PMID: 17220306 Free PMC article.
-
Impact of Helicobacter pylori infection on the humoral immune response to MUC1 peptide in patients with chronic gastric diseases and gastric cancer.Immunol Invest. 2007;36(4):371-86. doi: 10.1080/08820130601109727. Immunol Invest. 2007. PMID: 17691020
-
Helicobacter pylori-specific CD4+ T cells home to and accumulate in the human Helicobacter pylori-infected gastric mucosa.Infect Immun. 2005 Sep;73(9):5612-9. doi: 10.1128/IAI.73.9.5612-5619.2005. Infect Immun. 2005. PMID: 16113278 Free PMC article.
-
Enhanced T-helper 2 lymphocyte responses: immune mechanism of Helicobacter pylori infection.Ir J Med Sci. 1996 Jan-Mar;165(1):37-9. doi: 10.1007/BF02942800. Ir J Med Sci. 1996. PMID: 8867497 Review.
Cited by
-
Investigation of interleukin-10 promoter polymorphisms and interleukin-10 levels in children with irritable bowel syndrome.Gut Liver. 2013 Jul;7(4):430-6. doi: 10.5009/gnl.2013.7.4.430. Epub 2013 Jun 11. Gut Liver. 2013. PMID: 23898383 Free PMC article.
-
Lymphocyte proliferative response to Helicobacter pylori antigens in H. pylori-infected patients.Folia Microbiol (Praha). 2010 Nov;55(6):649-56. doi: 10.1007/s12223-010-0105-7. Epub 2011 Jan 21. Folia Microbiol (Praha). 2010. PMID: 21253914
-
Gut-brain Axis and migraine headache: a comprehensive review.J Headache Pain. 2020 Feb 13;21(1):15. doi: 10.1186/s10194-020-1078-9. J Headache Pain. 2020. PMID: 32054443 Free PMC article. Review.
-
Helicobacter pylori infection: Beyond gastric manifestations.World J Gastroenterol. 2020 Jul 28;26(28):4076-4093. doi: 10.3748/wjg.v26.i28.4076. World J Gastroenterol. 2020. PMID: 32821071 Free PMC article. Review.
-
Production and characterization of mouse monoclonal antibodies recognizing multiple subclasses of human IgG.Avicenna J Med Biotechnol. 2010 Jan;2(1):37-45. Avicenna J Med Biotechnol. 2010. PMID: 23408735 Free PMC article.
References
-
- Marshall BJ. Unidentified curved bacilli on the gastric epithelium in active chronic gastritis. Lancet 1983;I:1273–5. - PubMed
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;I:1311–15. - PubMed
-
- Bayerdorffer E , Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995;345:1591–4. - PubMed
-
- Ernst PB, Pecquet S. Interactions between Helicobacter pylori and the local mucosal immune system. Scand J Gastroenterol 1991;187:56–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
