CD4+ T cell mediated intestinal immunity: chronic inflammation versus immune regulation
- PMID: 15591505
- PMCID: PMC1774349
- DOI: 10.1136/gut.2003.037663
CD4+ T cell mediated intestinal immunity: chronic inflammation versus immune regulation
Abstract
Background: Several studies have suggested that chronic inflammatory bowel disease may be a consequence of antigen specific recognition by appropriate T cells which expand and induce immunopathology.
Aims: We wished to investigate whether autoreactive CD4+ T cells can initiate the disease on recognition of enterocyte specific antigens directly and if induction of mucosal tolerance occurs.
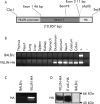
Methods: Transgenic mice (VILLIN-HA) were generated that showed specific expression of haemagglutinin from influenza virus A exclusively in enterocytes of the intestinal epithelium. To investigate the impact of enterocyte specific haemagglutinin expression in an autoimmune environment, we mated VILLIN-HA mice with T cell receptor (TCR)-HA mice expressing an alpha/beta-TCR, which recognises an MHC class II restricted epitope of haemagglutinin, and analysed the HA specific T cells for induction of autoimmunity or tolerance.
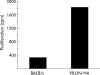
Results: In VILLIN-HAxTCR-HA mice, incomplete central deletion of HA specific lymphocytes occurred. Peripheral HA specific lymphocytes showed an activated phenotype and increased infiltration into the intestinal mucosa, but not into other organs of double transgenic mice. Enterocyte specific lamina propria lymphocytes showed a dose dependent proliferative response on antigen stimulation whereas the proliferative capacity of intraepithelial lymphocytes was reduced. Mucosal lymphocytes from VILLIN-HAxTCR-HA mice secreted lower amounts of interferon gamma and interleukin (IL)-2 but higher levels of tumour necrosis factor alpha, monocyte chemoattractant protein 1, and IL-6. Mucosal immune reactions were accompanied by broad changes in the gene expression profile with expression of proinflammatory genes, but strikingly also a remarkable set of genes discussed in the context of peripheral induction of regulatory T cells, including IL-10, Nrp-1, and Foxp3.
Conclusions: Enterocyte specific antigen expression is sufficient to trigger a specific CD4+ T cell response leading to mucosal infiltration. In our model, progression to overt clinical disease was counteracted most likely by induction of regulatory T cells.
Figures







Comment in
-
Immune regulation and colitis: suppression of acute inflammation allows the development of chronic inflammatory bowel disease.Gut. 2005 Jan;54(1):4-6. doi: 10.1136/gut.2004.047084. Gut. 2005. PMID: 15596416 Free PMC article. Review. No abstract available.
References
-
- Nagler-Anderson C . Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol 2001;1:59–67. - PubMed
-
- Kisielow P , Teh HS, Bluthmann H, et al. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature 1988;335:730–3. - PubMed
-
- Von Boehmer H , Aifantis I, Gounari F, et al. Thymic selection revisited: how essential is it? Immunol Rev 2003;191:62–78. - PubMed
-
- Melamed D , Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol 1993;23:935–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
