High frequency of functional anti-YMDD and -mutant cytotoxic T lymphocytes after in vitro expansion correlates with successful response to lamivudine therapy for chronic hepatitis B
- PMID: 15591521
- PMCID: PMC1774356
- DOI: 10.1136/gut.2003.032920
High frequency of functional anti-YMDD and -mutant cytotoxic T lymphocytes after in vitro expansion correlates with successful response to lamivudine therapy for chronic hepatitis B
Abstract
Background: Many determinants for a sustained response to lamivudine therapy have been reported but the role of T cell responsiveness remains unclear. The finding that tyrosine-methionine-aspartate-aspartate (YMDD) motif of the reverse transcriptase domain of hepatitis B virus (HBV) DNA polymerase carries a HLA-A2 restricted cytotoxic T lymphocyte (CTL) epitope makes quantitative measurement of the numbers of peptide specific CTLs feasible using MHC tetramer-peptide complex staining.
Aim: To investigate the correlation between anti-YMDD motif CTL activity and the efficacy of lamivudine therapy in HLA-A2 positive patients with chronic hepatitis B (CH-B).
Methods: The function and phenotype of peptide and interleukin 2 expanded peripheral blood mononuclear cells were quantified by cell lytic assay and immunocytochemical analysis by staining with HLA-A2-peptide tetramer complexes.
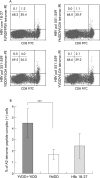
Results: After in vitro expansion, sustained responders had more potent CTL responses against YMDD, YVDD, and YIDD, as well as other epitopes on HBV antigens than non-responders. The frequency of YMDD/YVDD/YIDD motif specific CTLs increased significantly with an effective cell lytic function during and after therapy in sustained responders but not in non-responders. YMDD specific CTLs cross reacted with YIDD and YVDD mutant epitopes, and shared T cell receptor gene usages with YIDD and YVDD specific CTLs.
Conclusions: Sustained responders, at least HLA-A2 patients, elicited a more potent CTL immunity against YMDD and its mutants. YMDD specific CTLs are cross reactive with YVDD and YIDD mutant epitopes, which may further contribute to immune clearance of the mutant viruses and a successful response to lamivudine therapy in CH-B patients.
Figures




Comment in
-
Resistance to lamivudine therapy: is there more than meets the eye?Gut. 2005 Jan;54(1):9-10. doi: 10.1136/gut.2004.047548. Gut. 2005. PMID: 15591497 Free PMC article. Review. No abstract available.
References
-
- Lai CL, Chien RH, Leung NWY, et al. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med 1998;339:61–8. - PubMed
-
- Jarvis B , Faulds D. Lamivudine: a review of its therapeutic potential in chronic hepatitis B. Drugs 1999;58:101–41. - PubMed
-
- Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B. N Engl J Med 1999;341:1256–63. - PubMed
-
- Liaw YF, Leung NWY, Chang TT, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterology 2000;119:172–80. - PubMed
-
- Jonas MM, Kelley DA, Mizerski J, et al. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med 2002;346:1706–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
