Impaired mitochondrial glutamate transport in autosomal recessive neonatal myoclonic epilepsy
- PMID: 15592994
- PMCID: PMC1196378
- DOI: 10.1086/427564
Impaired mitochondrial glutamate transport in autosomal recessive neonatal myoclonic epilepsy
Abstract
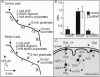
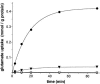
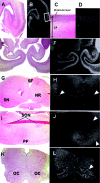
Severe neonatal epilepsies with suppression-burst pattern are epileptic syndromes with either neonatal onset or onset during the first months of life. These disorders are characterized by a typical electroencephalogram pattern--namely, suppression burst, in which higher-voltage bursts of slow waves mixed with multifocal spikes alternate with isoelectric suppression phases. Here, we report the genetic mapping of an autosomal recessive form of this condition to chromosome 11p15.5 and the identification of a missense mutation (p.Pro206Leu) in the gene encoding one of the two mitochondrial glutamate/H(+) symporters (SLC25A22, also known as "GC1"). The mutation cosegregated with the disease and altered a highly conserved amino acid. Functional analyses showed that glutamate oxidation in cultured skin fibroblasts from patients was strongly defective. Further studies in reconstituted proteoliposomes showed defective [(14)C]glutamate uniport and [(14)C]glutamate/glutamate exchange by mutant protein. Moreover, expression studies showed that, during human development, SLC25A22 is specifically expressed in the brain, within territories proposed to contribute to the genesis and control of myoclonic seizures. These findings provide the first direct molecular link between glutamate mitochondrial metabolism and myoclonic epilepsy and suggest potential insights into the pathophysiological bases of severe neonatal epilepsies with suppression-burst pattern.
Figures
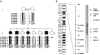
References
Electronic-Database Information
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for SLC25A22 cDNA [accession number NM_024698] and PAC clone RP13-569C6 [accession number AC132936])
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for nonketotic hyperglycinemia, propionic acidemia, type II citrullinemia, triple H syndrome, Amish microcephaly, adPEO, and Stanley syndrome)
-
- UCSC Genome Bioinformatics, http://genome.ucsc.edu/ (for the human genome working draft)
References
-
- Aicardi J (1992) Early myoclonic encephalopathy. In: Roger J, Bureau M, Dravet C, Dreifuss FE, Perret A, Wolf P (eds) Epileptic syndromes in infancy, childhood and adolescence, 2nd ed. John Libbey, London, pp 13–23
-
- Aicardi J, Goutières F (1978) [Neonatal myoclonic encephalopathy.] Rev Electroencephalogr Neurophysiol Clin 8:99–101 - PubMed
-
- Clements JR, Monaghan PL, Beitz AJ (1987) An ultrastructural description of glutamate-like immunoreactivity in the rat cerebellar cortex. Brain Res 421:343–348 - PubMed
-
- Degoul F, Diry M, Rodriguez D, Robain O, Francois D, Ponsot G, Marsac C, Desguerre I (1995) Clinical, biochemical, and molecular analysis of a maternally inherited case of Leigh syndrome (MILS) associated with the mtDNA T8993G point mutation. J Inherit Metab Dis 18:682–688 - PubMed
-
- Depaulis A, Vergnes M, Marescaux C (1994) Endogenous control of epilepsy: the nigral inhibitory system. Prog Neurobiol 42:33–52 - PubMed
Supplemental References
-
- Aubert S, Bligny R, Douce R, Gout E, Ratcliffe RG, Roberts JK (2001) Contribution of glutamate dehydrogenase to mitochondrial glutamate metabolism studied by 13C and 31P nuclear magnetic resonance. J Exp Bot 52:37–45 - PubMed
-
- Bourgeron T, Chretien D, Rotig A, Munnich A, Rustin P (1993) Fate and expression of the deleted mitochondrial DNA differ between human heteroplasmic skin fibroblast and Epstein-Barr virus–transformed lymphocyte cultures. J Biol Chem 268:19369–19376 - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 - PubMed
-
- Brines ML, Sundaresan S, Spencer DD, de Lanerolle NC (1997) Quantitative autoradiographic analysis of ionotropic glutamate receptor subtypes in human temporal lobe epilepsy: up-regulation in reorganized epileptogenic hippocampus. Eur J Neurosci 9:2035–2044 - PubMed
-
- Fiermonte G, Dolce V, Palmieri F (1998) Expression in Escherichia coli, functional characterization, and tissue distribution of isoforms A and B of the phosphate carrier from bovine mitochondria. J Biol Chem 273:22782–22787 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

