Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages
- PMID: 15595946
- PMCID: PMC2690188
- DOI: 10.1111/j.0887-378X.2004.00327.x
Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages
Erratum in
- Milbank Q. 2006;84(4):759-60
Abstract
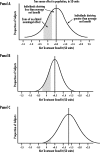
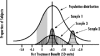
Evidence-based medicine is the application of scientific evidence to clinical practice. This article discusses the difficulties of applying global evidence ("average effects" measured as population means) to local problems (individual patients or groups who might depart from the population average). It argues that the benefit or harm of most treatments in clinical trials can be misleading and fail to reveal the potentially complex mixture of substantial benefits for some, little benefit for many, and harm for a few. Heterogeneity of treatment effects reflects patient diversity in risk of disease, responsiveness to treatment, vulnerability to adverse effects, and utility for different outcomes. Recognizing these factors, researchers can design studies that better characterize who will benefit from medical treatments, and clinicians and policymakers can make better use of the results.
Figures

References
-
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The Relationship of APOE Genotype to Neuropsychological Performance in Long-Term Cancer Survivors Treated with Standard Dose Chemotherapy. Psycho-oncology. 2003;12:612–9. 10.1002/pon.742. - DOI - PubMed
-
- B-Blocker Heart Attack Trial Research Group. A Randomized Trial of Propranolol in Patients with Acute Myocardial Infarction. I. Mortality Results. Journal of the American Medical Association. 1982;247:1707–14. 10.1001/jama.247.12.1707. - DOI - PubMed
-
- Baird KL. The New NIH and FDA Medical Research Policies: Targeting Gender, Promoting Justice. Journal of Health Politics, Policy and Law. 1999;24(3):531–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

