Electro-deformation and poration of giant vesicles viewed with high temporal resolution
- PMID: 15596488
- PMCID: PMC1305119
- DOI: 10.1529/biophysj.104.050310
Electro-deformation and poration of giant vesicles viewed with high temporal resolution
Abstract
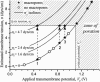
Fast digital imaging was used to study the deformation and poration of giant unilamellar vesicles subjected to electric pulses. For the first time the dynamics of response and relaxation of the membrane at micron-scale level is revealed at a time resolution of 30 micros. Above a critical transmembrane potential the lipid bilayer ruptures. Formation of macropores (diameter approximately 2 microm) with pore lifetime of approximately 10 ms has been detected. The pore lifetime has been interpreted as interplay between the pore edge tension and the membrane viscosity. The reported data, covering six decades of time, show the following regimes in the relaxation dynamics of the membrane. Tensed vesicles first relax to release the acquired stress due to stretching, approximately 100 micros. In the case of poration, membrane resealing occurs with a characteristic time of approximately 10 ms. Finally, for vesicles with excess area an additional slow regime was observed, approximately 1 s, which we associate with relaxation of membrane curvature. Dimensional analysis can reasonably well explain the corresponding characteristic timescales. Being performed on cell-sized giant unilamellar vesicles, this study brings insight to cell electroporation. The latter is widely used for gene transfection and drug transport across the membrane where processes occurring at different timescales may influence the efficiency.
Figures






References
-
- Abidor, I. G., V. B. Arakelyan, L. V. Chernomordik, Y. A. Chizmadzhev, V. F. Pastushenko, and M. R. Tarasevich. 1979. Electric breakdown of bilayer lipid membranes. I. The main experimental facts and their qualitative discussion. J. Electroanal. Chem. 104:37–52.
-
- Angelova, M. I., and D. S. Dimitrov. 1986. Liposome electroformation. Faraday Discuss. Chem. Soc. 81:303–311.
-
- Aranda-Espinoza, H., H. Bermudez, F. S. Bates, and D. E. Discher. 2001. Electromechanical limits of polymersomes. Phys. Rev. Lett. 20:208301. - PubMed
-
- Bermúdez, H., H. Aranda-Espinoza, D. A. Hammer, and D. E. Discher. 2003. Pore stability and dynamics in polymer membranes. Europhys. Lett. 64:550–556.
-
- Brochard-Wyart, F., P. G. de Gennes, and O. Sandre. 2000. Transient pores in stretched vesicles: role of leak-out. Physica A. 278:32–51.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

