Skeletal muscle NAD(P)H two-photon fluorescence microscopy in vivo: topology and optical inner filters
- PMID: 15596503
- PMCID: PMC1305268
- DOI: 10.1529/biophysj.104.053165
Skeletal muscle NAD(P)H two-photon fluorescence microscopy in vivo: topology and optical inner filters
Abstract
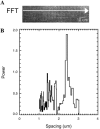
Two-photon excitation fluorescence microscopy (TPEFM) permits the investigation of the topology of intercellular events within living animals. TPEFM was used to monitor the distribution of mitochondrial reduced nicotinamide adenine dinucleotide (NAD(P)H) in murine skeletal muscle in vivo. NAD(P)H fluorescence emission was monitored ( approximately 460 nm) using 710-720 nm excitation. High-resolution TPEFM images were collected up to a depth of 150 microm from the surface of the tibialis anterior muscle. The NAD(P)H fluorescence images revealed subcellular structures consistent with subsarcolemmal, perivascular, intersarcomeric, and paranuclear mitochondria. In vivo fiber typing between IIB and IIA/D fibers was possible using the distribution and content of mitochondria from the NAD(P)H fluorescence signal. The intersarcomeric mitochondria concentrated at the Z-line in the IIB fiber types resulting in a periodic pattern with a spacing of one sarcomere (2.34 +/- 0.17 microm). The primary inner filter effects were nearly equivalent to water, however, the secondary inner filter effects were highly significant and dynamically affected the observed emission frequency and amplitude of the NAD(P)H fluorescence signal. These data demonstrate the feasibility, and highlight the complexity, of using NAD(P)H TPEFM in skeletal muscle to characterize the topology and metabolic function of mitochondria within the living mouse.
Figures






Similar articles
-
NAD(P)H fluorescence imaging of mitochondrial metabolism in contracting Xenopus skeletal muscle fibers: effect of oxygen availability.J Appl Physiol (1985). 2005 Apr;98(4):1420-6. doi: 10.1152/japplphysiol.00849.2004. Epub 2004 Dec 10. J Appl Physiol (1985). 2005. PMID: 15591295
-
Optical imaging and functional characterization of the transverse tubular system of mammalian muscle fibers using the potentiometric indicator di-8-ANEPPS.J Membr Biol. 2005 Nov;208(2):141-53. doi: 10.1007/s00232-005-0825-9. J Membr Biol. 2005. PMID: 16645743
-
Functional imaging of mitochondria in saponin-permeabilized mice muscle fibers.J Cell Biol. 1998 Mar 9;140(5):1091-9. doi: 10.1083/jcb.140.5.1091. J Cell Biol. 1998. PMID: 9490722 Free PMC article.
-
Multiphoton FLIM imaging of NAD(P)H and FAD with one excitation wavelength.J Biomed Opt. 2020 Jan;25(1):1-16. doi: 10.1117/1.JBO.25.1.014510. J Biomed Opt. 2020. PMID: 31920048 Free PMC article.
-
NAD+ : A big player in cardiac and skeletal muscle remodeling and aging.J Cell Physiol. 2018 Mar;233(3):1895-1896. doi: 10.1002/jcp.26014. Epub 2017 Jul 7. J Cell Physiol. 2018. PMID: 28518407 Free PMC article. Review.
Cited by
-
Role of p110a subunit of PI3-kinase in skeletal muscle mitochondrial homeostasis and metabolism.Nat Commun. 2019 Jul 30;10(1):3412. doi: 10.1038/s41467-019-11265-y. Nat Commun. 2019. PMID: 31363081 Free PMC article.
-
Short communication: Subcellular motion compensation for minimally invasive microscopy, in vivo: evidence for oxygen gradients in resting muscle.Circ Res. 2010 Apr 2;106(6):1129-33. doi: 10.1161/CIRCRESAHA.109.211946. Epub 2010 Feb 18. Circ Res. 2010. PMID: 20167928 Free PMC article.
-
Quantitative second harmonic generation imaging of the diseased state osteogenesis imperfecta: experiment and simulation.Biophys J. 2008 Jun;94(11):4504-14. doi: 10.1529/biophysj.107.114405. Epub 2008 Feb 15. Biophys J. 2008. PMID: 18281387 Free PMC article.
-
Limited utility of acetoxymethyl (AM)-based intracellular delivery systems, in vivo: interference by extracellular esterases.J Microsc. 2007 Apr;226(Pt 1):74-81. doi: 10.1111/j.1365-2818.2007.01755.x. J Microsc. 2007. PMID: 17381712 Free PMC article.
-
In vivo microscopy reveals extensive embedding of capillaries within the sarcolemma of skeletal muscle fibers.Microcirculation. 2014 Feb;21(2):131-47. doi: 10.1111/micc.12098. Microcirculation. 2014. PMID: 25279425 Free PMC article.
References
-
- Avi-Dor, Y., E. Lamdin, and N. O. Kaplan. 1963. Structural factors in the succinate-induced reduction of mitochondrial pyridine nucleotides. J. Biol. Chem. 238:2518–2528. - PubMed
-
- Balaban, R. S. 2002. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J. Mol. Cell. Cardiol. 34:1259–1271. - PubMed
-
- Blinova, K., S. Carroll, S. Bose, A. V. Smirnov, J. J. Harvey, J. R. Knutson, and R. S. Balaban. 2004. The distribution of mitochondrial NADH fluorescence lifetimes: steady state kinetics of matrix NADH interactions. Biochemistry. In press. - PubMed
-
- Bose, S., S. French, F. J. Evans, F. Joubert, and R. S. Balaban. 2003. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J. Biol. Chem. 278:39155–39165. - PubMed
-
- Brandes, R., V. M. Figueredo, S. A. Camacho, and M. W. Weiner. 1994. Compensation for changes in tissue light absorption in fluorometry of hypoxic perfused rat hearts. Am. J. Physiol. 266:H2554–H2567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

