Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores
- PMID: 15596510
- PMCID: PMC1305237
- DOI: 10.1529/biophysj.104.049817
Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores
Abstract
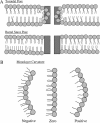
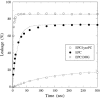
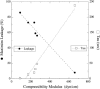
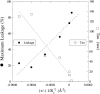
The membrane-lytic peptide melittin has previously been shown to form pores in lipid bilayers that have been described in terms of two different structural models. In the "barrel stave" model the bilayer remains more or less flat, with the peptides penetrating across the bilayer hydrocarbon region and aggregating to form a pore, whereas in the "toroidal pore" melittin induces defects in the bilayer such that the bilayer bends sharply inward to form a pore lined by both peptides and lipid headgroups. Here we test these models by measuring both the free energy of melittin transfer (DeltaG degrees ) and melittin-induced leakage as a function of bilayer elastic (material) properties that determine the energetics of bilayer bending, including the area compressibility modulus (K(a)), bilayer bending modulus (k(c)), and monolayer spontaneous curvature (R(o)). The addition of cholesterol to phosphatidylcholine (PC) bilayers, which increases K(a) and k(c), decreases both DeltaG degrees and the melittin-induced vesicle leakage. In contrast, the addition to PC bilayers of molecules with either positive R(o), such as lysoPC, or negative R(o), such as dioleoylglycerol, has little effect on DeltaG degrees , but produces large changes in melittin-induced leakage, from 86% for 8:2 PC/lysoPC to 18% for 8:2 PC/dioleoylglycerol. We observe linear relationships between melittin-induced leakage and both K(a) and 1/R(o)(2). However, in contrast to what would be expected for a barrel stave model, there is no correlation between observed leakage and bilayer hydrocarbon thickness. All of these results demonstrate the importance of bilayer material properties on melittin-induced leakage and indicate that the melittin-induced pores are defects in the bilayer lined in part by lipid molecules.
Figures

References
-
- Allende, D., and T. J. McIntosh. 2003. Lipopolysaccharides in bacterial membranes act like cholesterol in eukaryotic plasma membranes in providing protection against melittin-induced bilayer lysis. Biochemistry. 42:1101–1108. - PubMed
-
- Allende, D., A. Vidal, S. A. Simon, and T. J. McIntosh. 2003. Bilayer interfacial properties modulate the binding of amphipathic peptides. Chem. Phys. Lipids. 122:65–76. - PubMed
-
- Barranger-Mathys, M., and D. S. Cafiso. 1996. Membrane structure of voltage-gated channel forming peptides revealed by site-directed spin labeling. Biochemistry. 35:498–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

