Fluorescence imaging for monitoring the colocalization of two single molecules in living cells
- PMID: 15596511
- PMCID: PMC1305264
- DOI: 10.1529/biophysj.104.048967
Fluorescence imaging for monitoring the colocalization of two single molecules in living cells
Abstract
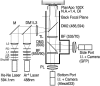
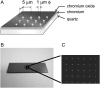
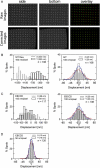
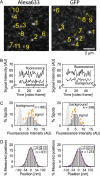
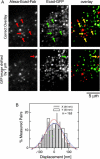
The interaction, binding, and colocalization of two or more molecules in living cells are essential aspects of many biological molecular processes, and single-molecule technologies for investigating these processes in live cells, if successfully developed, would become very powerful tools. Here, we developed simultaneous, dual-color, single fluorescent molecule colocalization imaging, to quantitatively detect the colocalization of two species of individual molecules. We first established a method for spatially correcting the two full images synchronously obtained in two different colors, and then for overlaying them with an accuracy of 13 nm. By further assessing the precision of the position determination, and the signal/noise and signal/background ratios, we found that two single molecules in dual color can be colocalized to within 64-100 nm (68-90% detectability) in the membrane of cells for GFP and Alexa633. The detectability of true colocalization at the molecular level and the erroneous inclusion of incidental approaches of two molecules as colocalization have to be compromised at different levels in each experiment, depending on its purpose. This technique was successfully demonstrated in living cells in culture, monitoring colocalization of single molecules of E-cadherin fused with GFP diffusing in the plasma membrane with single molecules of Alexa633 conjugated to anti-E-cadherin Fab externally added to the culture medium. This work established a benchmark for monitoring the colocalization of two single molecules, which can be applied to wide ranges of studies for molecular interactions, both at the levels of single molecules and collections of molecules.
Figures


Comment in
-
DySCo: quantitating associations of membrane proteins using two-color single-molecule tracking.Biophys J. 2009 Aug 19;97(4):L5-7. doi: 10.1016/j.bpj.2009.05.046. Biophys J. 2009. PMID: 19686638 Free PMC article.
References
-
- Anderson, C. M., G. N. Georgiou, I. E. Morrison, G. V. Stevenson, and R. J. Cherry. 1992. Tracking of cell surface receptors by fluorescence digital imaging microscopy using a charge-coupled device camera. Low-density lipoprotein and influenza virus receptor mobility at 4 degrees C. J. Cell Sci. 101:415–425. - PubMed
-
- Chang, C. H., H. Takeuchi, T. Ito, K. Machida, and S. Ohnishi. 1981. Lateral mobility of erythrocyte membrane proteins studied by the fluorescence photobleaching recovery technique. J. Biochem. (Tokyo). 90:997–1004. - PubMed
-
- Derrick, J. P., and D. B. Wigley. 1994. The third IgG-binding domain from streptococcal protein G. An analysis by X-ray crystallography of the structure alone and in a complex with Fab. J. Mol. Biol. 243:906–918. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

