The mechanism of amyloid spherulite formation by bovine insulin
- PMID: 15596515
- PMCID: PMC1305253
- DOI: 10.1529/biophysj.104.051896
The mechanism of amyloid spherulite formation by bovine insulin
Abstract
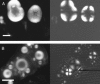
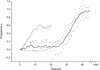
The formation of amyloid-containing spherulite-like structures has been observed in some instances of amyloid diseases, as well as in amyloid fibril-containing solutions in vitro. In this article we describe the structure and kinetics of bovine insulin amyloid fibril spherulites formed in the presence and absence of different salts and at different salt concentrations. The general spherulite structure consists of radially oriented amyloid fibrils, as shown by optical microscopy and environmental scanning electron microscopy. In the center of each spherulite, a "core" of less regularly oriented material is observed, whose size decreases when the spherulites are formed in the presence of increasing concentrations of NaCl. Similarly, amyloid fibrils form faster in the presence of NaCl than in its absence. A smaller enhancement of the rate of formation with salt concentration is observed for spherulites. These data suggest that both amyloid fibril formation and random aggregation occur concurrently under the conditions tested. Changes in their relative rates result in the different-sized cores observed in the spherulites. This mechanism can be likened to that leading to the formation of spherulites of polyethylene, in agreement with observations that polypeptide chains under partially denaturing conditions can exhibit behavior not dissimilar to that of synthetic polymers.
Figures




Similar articles
-
Optical microscopy of growing insulin amyloid spherulites on surfaces in vitro.Biophys J. 2006 Feb 1;90(3):1043-54. doi: 10.1529/biophysj.105.072660. Epub 2005 Nov 4. Biophys J. 2006. PMID: 16272436 Free PMC article.
-
Kinetics of spherulite formation and growth: salt and protein concentration dependence on proteins beta-lactoglobulin and insulin.Int J Biol Macromol. 2009 May 1;44(4):301-10. doi: 10.1016/j.ijbiomac.2008.12.014. Int J Biol Macromol. 2009. PMID: 19437593
-
Factors affecting the formation of insulin amyloid spherulites.Colloids Surf B Biointerfaces. 2012 Jan 1;89:216-22. doi: 10.1016/j.colsurfb.2011.09.018. Epub 2011 Sep 17. Colloids Surf B Biointerfaces. 2012. PMID: 21982213
-
Protein aggregation: more than just fibrils.Biochem Soc Trans. 2009 Aug;37(Pt 4):682-6. doi: 10.1042/BST0370682. Biochem Soc Trans. 2009. PMID: 19614575 Review.
-
The route to protein aggregate superstructures: Particulates and amyloid-like spherulites.FEBS Lett. 2015 Sep 14;589(19 Pt A):2448-63. doi: 10.1016/j.febslet.2015.07.006. Epub 2015 Jul 14. FEBS Lett. 2015. PMID: 26183565 Review.
Cited by
-
Protein disorder-order interplay to guide the growth of hierarchical mineralized structures.Nat Commun. 2018 Jun 1;9(1):2145. doi: 10.1038/s41467-018-04319-0. Nat Commun. 2018. PMID: 29858566 Free PMC article.
-
Liquid-crystalline nanoarchitectures for tissue engineering.Beilstein J Nanotechnol. 2018 Jan 18;9:205-215. doi: 10.3762/bjnano.9.22. eCollection 2018. Beilstein J Nanotechnol. 2018. PMID: 29441265 Free PMC article. Review.
-
Characterization of heat induced spherulites of lysozyme reveals new insight on amyloid initiation.Sci Rep. 2016 Mar 1;6:22475. doi: 10.1038/srep22475. Sci Rep. 2016. PMID: 26926993 Free PMC article.
-
Cooperative self-assembly of peptide gelators and proteins.Biomacromolecules. 2013 Dec 9;14(12):4368-76. doi: 10.1021/bm401319c. Epub 2013 Nov 27. Biomacromolecules. 2013. PMID: 24256076 Free PMC article.
-
Self-propagating beta-sheet polypeptide structures as prebiotic informational molecular entities: the amyloid world.Orig Life Evol Biosph. 2009 Apr;39(2):141-50. doi: 10.1007/s11084-009-9165-6. Epub 2009 Mar 20. Orig Life Evol Biosph. 2009. PMID: 19301141
References
-
- Acebo, E., M. Mayorga, and J. F. Val-Bernal. 1999. Primary amyloid tumor (amyloidoma) of the jejunum with spheroid type of amyloid. Pathol. 31:8–11. - PubMed
-
- Aggeli, A., M. Bell, L. M. Carrick, C. W. G. Fishwick, R. Harding, P. J. Mawer, S. E. Radford, A. E. Strong, and N. Boden. 2003. pH as a trigger of peptide β-sheet self-assembly and reversible switching between nematic and isotropic phases. J. Am. Chem. Soc. 125:9619–9628. - PubMed
-
- Aggeli, A., G. Fytas, D. Vlassopoulos, T. C. B. McLeish, P. J. Mawer, and N. Boden. 2001. Structure and dynamics of self-assembling β-sheet peptide tapes by dynamic light scattering. Biomacromolecules. 2:378–388. - PubMed
-
- Ahmad, A., I. S. Millett, S. Doniach, V. N. Uversky, and A. L. Fink. 2003. Partially folded intermediates in insulin fibrillation. Biochemistry. 42:11404–11416. - PubMed
-
- Bassett, D. C. 2003. Polymer spherulites: a modern assessment. J. Macromol. Sci. Phys. 42:227–256.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

