Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation
- PMID: 15596520
- PMCID: PMC2211994
- DOI: 10.1084/jem.20031958
Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation
Abstract
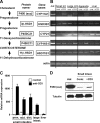
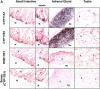
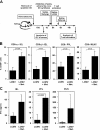
Glucocorticoids (GCs) are important steroid hormones with widespread activities in metabolism, development, and immune regulation. The adrenal glands are the major source of GCs and release these hormones in response to psychological and immunological stress. However, there is increasing evidence that GCs may also be synthesized by nonadrenal tissues. Here, we report that the intestinal mucosa expresses steroidogenic enzymes and releases the GC corticosterone in response to T cell activation. T cell activation causes an increase in the intestinal expression of the steroidogenic enzymes required for GC synthesis. In situ hybridization analysis revealed that these enzymes are confined to the crypt region of the intestinal epithelial layer. Surprisingly, in situ-produced GCs exhibit both an inhibitory and a costimulatory role on intestinal T cell activation. In the absence of intestinal GCs in vivo, activation by anti-CD3 injection resulted in reduced CD69 expression and interferon-gamma production by intestinal T cells, whereas activation by viral infection led to increased T cell activation. We conclude that the intestinal mucosa is a potent source of immunoregulatory GCs.
Figures









Similar articles
-
The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine.J Exp Med. 2006 Sep 4;203(9):2057-62. doi: 10.1084/jem.20060357. Epub 2006 Aug 21. J Exp Med. 2006. PMID: 16923850 Free PMC article.
-
Colon cancer cells produce immunoregulatory glucocorticoids.Oncogene. 2011 May 26;30(21):2411-9. doi: 10.1038/onc.2010.629. Epub 2011 Jan 24. Oncogene. 2011. PMID: 21258413
-
Comprehensive phenotypic analysis of the gut intra-epithelial lymphocyte compartment: perturbations induced by acute reovirus 1/L infection of the gastrointestinal tract.Int Immunol. 2007 Apr;19(4):567-79. doi: 10.1093/intimm/dxm022. Epub 2007 Mar 15. Int Immunol. 2007. PMID: 17369189
-
Extra-Adrenal Glucocorticoid Synthesis in the Intestinal Mucosa: Between Immune Homeostasis and Immune Escape.Front Immunol. 2019 Jun 25;10:1438. doi: 10.3389/fimmu.2019.01438. eCollection 2019. Front Immunol. 2019. PMID: 31316505 Free PMC article. Review.
-
Control of immunity by glucocorticoids in health and disease.Semin Immunopathol. 2020 Dec;42(6):669-680. doi: 10.1007/s00281-020-00827-8. Epub 2020 Nov 20. Semin Immunopathol. 2020. PMID: 33219395 Review.
Cited by
-
Macrolides increase the expression of 11β-hydroxysteroid dehydrogenase 1 in human sinonasal epithelium, contributing to glucocorticoid activation in sinonasal mucosa.Br J Pharmacol. 2015 Nov;172(21):5083-95. doi: 10.1111/bph.13273. Epub 2015 Oct 17. Br J Pharmacol. 2015. PMID: 26228509 Free PMC article.
-
Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function.Am J Physiol Endocrinol Metab. 2011 Jul;301(1):E11-24. doi: 10.1152/ajpendo.00100.2011. Epub 2011 May 3. Am J Physiol Endocrinol Metab. 2011. PMID: 21540450 Free PMC article. Review.
-
Local glucocorticoid synthesis regulates house dust mite-induced airway hypersensitivity in mice.Front Immunol. 2023 Oct 23;14:1252874. doi: 10.3389/fimmu.2023.1252874. eCollection 2023. Front Immunol. 2023. PMID: 37936704 Free PMC article.
-
Post-finasteride syndrome: An emerging clinical problem.Neurobiol Stress. 2019 Dec 26;12:100209. doi: 10.1016/j.ynstr.2019.100209. eCollection 2020 May. Neurobiol Stress. 2019. PMID: 32435662 Free PMC article.
-
Mouse testicular macrophages can independently produce testosterone and are regulated by Cebpb.Biol Res. 2024 Sep 9;57(1):64. doi: 10.1186/s40659-024-00544-8. Biol Res. 2024. PMID: 39252136 Free PMC article.
References
-
- Powrie, F. 1995. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 3:171–174. - PubMed
-
- Smith, K.M., A.D. Eaton, L.M. Finlayson, and P. Garside. 2000. Oral tolerance. Am. J. Respir. Crit. Care Med. 162:S175–S178. - PubMed
-
- Husband, A.J., K.W. Beagley, and J.R. McGhee. 1999. Mucosal cytokines. Mucosal Immunology. J.R. McGhee, editor. Academic Press, San Diego, CA. 541–557.
-
- Maloy, K.J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816–822. - PubMed
-
- Groux, H., and F. Powrie. 1999. Regulatory T cells and inflammatory bowel disease. Immunol. Today. 20:442–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

