Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin
- PMID: 15596525
- PMCID: PMC2211990
- DOI: 10.1084/jem.20041447
Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin
Abstract
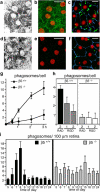
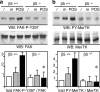
Daily phagocytosis by the retinal pigment epithelium (RPE) of spent photoreceptor outer segment fragments is critical for vision. In the retina, early morning circadian photoreceptor rod shedding precedes synchronized uptake of shed photoreceptor particles by RPE cells. In vitro, RPE cells use the integrin receptor alphavbeta5 for particle binding. Here, we tested RPE phagocytosis and retinal function in beta5 integrin--deficient mice, which specifically lack alphavbeta5 receptors. Retinal photoresponses severely declined with age in beta5-/- mice, whose RPE accumulated autofluorescent storage bodies that are hallmarks of human retinal aging and disease. beta5-/- RPE in culture failed to take up isolated photoreceptor particles. beta5-/- RPE in vivo retained basal uptake levels but lacked the burst of phagocytic activity that followed circadian photoreceptor shedding in wild-type RPE. Rhythmic activation of focal adhesion and Mer tyrosine kinases that mediate wild-type retinal phagocytosis was also completely absent in beta5-/- retina. These results demonstrate an essential role for alphavbeta5 integrin receptors and their downstream signaling pathways in synchronizing retinal phagocytosis. Furthermore, they identify the beta5-/- integrin mouse strain as a new animal model of age-related retinal dysfunction.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

